SUMMARY
The lymphocyte profile of 521 HIV-infected subjects hospitalized at Jackson Memorial (2001–2002) was compared across main respiratory diseases. Study data included medical history and all laboratory evaluations performed during hospitalization. Community-acquired pneumonias (CAP, 52%), Pneumocystis jiroveci pneumonia (PCP, 24%), tuberculosis (TB, 9%) and non-tuberculous mycobacterial diseases (NTM, 12%) were the most frequent causes of admission. Patients hospitalized with PCP and NTM exhibited the lowest CD4 counts (P=0·003). PCP patients had the highest B-cell percentages (P=0·04). CAP patients had the highest CD8 and CD4 percentages and the lowest percentage of Natural Killer (NK) cells and viral burdens. TB patients exhibited the lowest NK-cell (11·4±6·3) and B-cell percentages (13·6±12) and the highest CD8 (59±14) percentage. NTM patients, in contrast, had the highest NK-cell percentages of the groups (19·1±11·6, P=0·01). Additionally, immune responses associated with respiratory pathogens differed in HIV-infected patients with CD4+ cells above and below 200 counts.
INTRODUCTION
The lungs are perhaps most challenged by the attack of microbial pathogens, requiring the immune response to be rapid and efficient. Despite great advances regarding immune response, its interaction with different pathogens and immune selection remains puzzling, particularly in HIV disease. Although the introduction of highly active antiretroviral therapy (HAART) has substantially reduced the prevalence of opportunistic infections, respiratory diseases continue to have a significant impact upon morbidity and mortality [1–3]. It is also important to be aware that immune improvement resulting from HAART is widely variable and greatly depends on how early HAART is implemented [4, 5].
Many questions remain as to why some HIV-infected individuals can control antigen challenges and remain relatively ‘healthy’, while others develop repetitive respiratory infections. Also unanswered is the issue of differential susceptibility among immunosuppressed individuals, some of whom seem more prone than others to certain respiratory diseases. A better understanding of the immune mechanisms involved in counteracting pathogens may evolve from studies comparing the immune responses of patients with different lower respiratory infections at diverse CDC stages [6] to those of HIV-infected subjects without respiratory infections. This information will undoubtedly be advantageous in the development of new, effective treatments against respiratory pathogens.
There has been extensive discussion as to whether or not local or systemic immune evaluations are appropriate when studying respiratory infections. While some scientists have argued that a local immune response needs to be assessed and is the only valid evaluation, others have countered that systemic compromise is also critically important. We certainly agree that non-specific immune response such as epithelial abnormalities, mucosal humoral defences and phagocytic responses needs to be measured locally. Nonetheless, once processing and presentation are completed a population of specific cells is expanded and the effector cells [T, B and Natural Killer (NK) cells] enter the lymphatic and circulation system. In certain diseases such as sepsis, cancer and HIV, the systemic component (imbalance of pro-inflammatory interleukins and oxidative stress) is mainly responsible for the impaired clearance of pathogens. In HIV disease, it is irrefutable that compromised systemic host defences impair the clearance of microbial pathogens from sites of tissue infection, such as the respiratory airways. Therefore, systemic evaluation is an appropriate research model and was used to characterize the immune response of HIV-infected patients hospitalized with respiratory infections.
METHODS
Study population
Eligible participants were adults aged 18 years and older, with confirmed HIV infection and who were consecutively admitted at the University of Miami/Jackson Memorial (2001–2003). Patients were fully informed about the study and those who signed the consent/medical release forms were enrolled. The University of Miami Institutional Review Board approved the study.
Medical history
Past medical history, and CDC AIDS defining criteria [6] were carefully documented. Once research procedures were completed, a medical chart was abstracted and three experienced HIV clinician/researchers compared and validated patients’ information with medical records. When a discrepancy was encountered medical records were usually considered to be the truthful answer. A detailed medication history, including antiretrovirals and prophylaxis was obtained.
HIV disease status and immune assessment
Isolated peripheral blood mononuclear cells were obtained within 72 h of admission and prepared for four-colour direct immunofluorecence procedures (Becton Dickinson, San Jose, CA, USA). Flow cytometry was used to quantify the percentage and absolute numbers of T lymphocyte subpopulations CD3+/CD4+, CD3+/CD8+, B lymphocytes (CD3+/CD19+) and NK cells (CD3+/CD56+).
A reverse transcriptase polymerase chain reaction was used to quantify HIV-1 viral load (Amplicor HIV-1 Monitor, Roche Diagnostics, Indianapolis, IN, USA) in plasma from ethylenediamine tetraacetic acid (EDTA)-collected blood samples that were separated and processed within 6 h of collection. The current version of this assay has a reportable range of >200–750 000 RNA copies/mm of plasma.
Hospitalization
Computerized diagnostic code data included: number of previous admissions to the hospital, date of present admission, length of stay, and presumptive admission diagnosis. Once the patient was discharged from the hospital, data coding included the main discharge diagnosis and a list of diagnostic examinations and procedures.
Routine clinical tests ordered by the physicians and available for analyses included: complete blood count, biochemistry tests, urinalysis, Gram stains, and blood cultures. Additional clinical diagnostic tests were ordered at the discretion of the treating physicians and were included in the database.
Respiratory infections
A diagnosis of respiratory infection required a combination of three or more of the following criteria: (1) medical diagnosis, (2) X-ray findings, (3) a positive culture, (4) isolation/visualization of the organism in sputum or bronchial wash and, (5) response to treatment. Diagnosis was established by the isolation of the organism in blood, sputum or bronchial wash and response to treatment.
A specific diagnosis for community-acquired pneumonia (CAP) was based on the Infectious Disease Society of America criteria [7] incorporating: (1) history and physical examination, (2) chest radiography, (3) sputum Gram stain and culture and, when necessary, bronchoscopy/biopsy.
Pneumocystis jiroveci pneumonia (PCP). A clinical diagnosis was considered if at least three of the following clinical criteria were present: (1) dyspnoea, (2) severe hypoxia (cyanosis or oxygen saturation <86%, as measured by pulse oximetry) and a lactate dehydrogenase level of >1000 U/l, (3) thorax X-ray compatible with PCP, (4) PCP identification or biopsy in which the pathologist suggested PCP or, (5) response to therapy.
Diagnosis of mycobacterial disease was established by isolation and culture of Mycobacteria. Non-tuberculous mycobacterial (NTM) pulmonary diagnosis was achieved when the American Thoracic Society (ATS) criteria were satisfied by: (a) underlying conditions (immunosuppression), (b) clinical (signs/symptoms) and radiographic studies (X-ray or CT scan), (c) bacteriological criteria (at least two distinct positive sputum cultures, or isolation in a bronchial wash or tissue biopsy cultures), or (d) when there was no alternative explanation for the clinical picture.
Participants were included in the non-respiratory infection category if the admission diagnosis did not indicate any pulmonary disease or development of respiratory illness during hospitalization (i.e. renal, CNS or genitourinary problems).
Statistical analyses
The data were analysed using SAS version 8 (SAS Institute, Cary, NC, USA) and SPSS version 11 (SPSS Inc., Chicago, IL, USA). Following descriptive statistical analyses, mean variables were compared using Student’s t test and one-way ANOVA procedures. Correlations between the main variables of interest were examined with Pearson’s correlation coefficient analyses. The final discharge diagnosis was used to classify patients into different study groups as follows: (1) CAP, (2) PCP, (3) tuberculosis (TB), (4) NTM, and (5) non-respiratory causes. Participants were also subdivided into three CD4 strata according to CDC classification: <200, 200–500 and >500 cells/mm3 [8].
Regression analyses were used to evaluate the relationships between discharge diagnosis, risk factors, immune function and antiretrovirals.
RESULTS
Study population
Sociodemographic characteristics
The 521 HIV-infected subjects included 300 men (58%) and 221 women (42%) who ranged in age from 20 to 72 years (42±9 years). More than half (61%) of the respondents self-identified as African American, 18% were Hispanic, 17% Haitian and 4% Caucasian. Years of education varied across the group from 1 to 18 years (9±4 years); 15% had >12 years of education.
HIV CDC classification, immune status, and HIV treatment
According to CDC criteria, most of the participants (80%) had AIDS and the remaining 20% were HIV symptomatic. The mean CD4 counts of the total group was 131±177; only 33% had >200 cells/mm3. The mean viral load was 260 090±293 508, with 17% having undetectable viral load levels (<400). No significant difference in CD4 counts were observed between patients hospitalized with respiratory infections (116±163 cells/mm3) and those hospitalized for other reasons (141±179, P=0·2).
Forty per cent of the study group was prescribed, and reported using HAART. Only 42% of the participants were taking trimethoprim–sulphamethoxazole and/or other prophylaxis. HAART-treated participants had significantly higher CD4 cell counts (168·5±216·3) compared to those without HAART (115±154, P=0·05), and lower HIV viral loads (treated 79 397±11 496 vs. non-treated 314 145±309 164 HIV copies/ml, P=0·0001). Although viral loads were still detectable, these did not reflect poor adherence, but rather the extremely high viral burden the patients had at the time of initiating HAART (>750 000 copies/ml). All these patients demonstrated a significant decrease in viral load, indicating they were in fact taking HIV medications. In five cases, antiretroviral resistance was documented later on and resulted in high viral burdens.
Discharge diagnosis
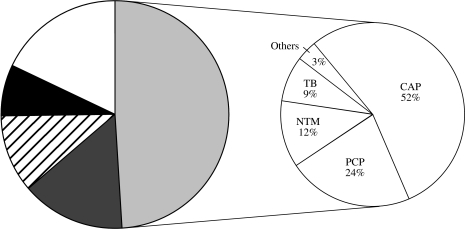
As shown in the Figure, respiratory diseases accounted for 49% of the total admissions, followed by gastrointestinal diseases (16%), central nervous system disorders (11%) and renal insufficiency (8%). The remaining 16% were similarly distributed among cardiovascular diseases, sepsis, genitourinary conditions, malignancy and a small per cent (1–2%) of traumas.
Fig.
Distribution of the frequent causes of hospitalization. , Respiratory diseases;
, Respiratory diseases;  , neurological diseases; □, others; ■, gastrointestinal diseases;
, neurological diseases; □, others; ■, gastrointestinal diseases;  , renal insufficiency.
, renal insufficiency.
CAP was the main cause of respiratory illnesses (52%, n=251) and hospital admissions. Pneumocystis jiroveci continued to be an important cause of respiratory infection (24%, n=65). In 21% of the study participants, mycobacteria were cultured in 9%, and NTM disease was evident in the remaining 12% of cases. For the remaining 3% discharge diagnosis was bronchitis/croup or asthma.
CD4 cell counts and respiratory infections of the lower respiratory tract
PCP and NTM cases were clustered in the stratum with the lowest CD4 counts (67·8±91, 59±47 respectively). PCP diagnosis was correlated with low CD4 cell counts (r=0·125, P=0·004) and was twice as common among patients with lower CD4 lymphocyte counts (<200 cells/μl) than among those with higher counts (P=0·002).
Univariate analyses indicated that patients with <200 CD4 cells were three times more likely to develop mycobacterial disease than any other respiratory disease except for PCP (95% CI 1·07–11·7, P=0·02). Participants with <200 cells were also twice as likely to develop a NTM disease, but not TB. No significant difference in the risk of developing CAP was observed in relationship to CD4 counts above or below 200 cells/mm3.
HAART, viral load and respiratory infections of the lower respiratory tract
In the non-HAART-treated group, viral load values were highest in patients with PCP and NTM, but not significantly different than measurements in patients with CAP, TB, or a non-respiratory illness.
For the HAART-treated group, viral burden was highest in NTM patients (159 634±43 285), followed by PCP (90 110±21 645) and the TB group (86 594±77 394). The lowest viral burden was seen in patients with CAP (75 233±21 645) and in the non-respiratory disease group (65 542±16 506), regardless of HAART. Among those receiving antiretrovirals, differences were noted between the NTM and CAP groups (P=0·08) as well as, the NTM and the non-respiratory group (P=0·04).
Phenotypic characterization of peripheral lymphocytes at diagnosis other than CD4
As shown in Table 1, a significantly lower percentage of CD3+ lymphocytes was evident in patients hospitalized with PCP compared to those with CAP (62·8±19 vs. 71·2±14·1, P=0·02). CD8 percentages were similar in these two groups (PCP 52·6±17·2 vs. CAP 55·2±15·5). The percentages of B and NK cells, in contrast, were significantly higher in PCP than in CAP patients (B cells 20·2±16·9 vs. 15·9±11·3, P=0·04; NK cells 16·8±10 vs. 13·0±9·3, P=0·02). Moreover, of all the groups, PCP patients had the highest B-cell percentages (P=0·04), the lowest CD4 counts, and the highest viral loads (P=0·01) at the time of diagnosis. CAP patients were characterized as having the highest CD4 percentages and counts and the lowest viral burden.
Table 1.
Lymphocyte immunophenotype according to respiratory diseases in HIV/AIDS

Bold figures highlight the highest and lowest values across the different respiratory diseases.
TB patients exhibited the highest CD8 percentage, and the lowest NK-cell (11·4±6·3, P=0·04) and B-cell percentages (11·5±7) across the study. NTM patients, in contrast, had the highest NK-cell percentages compared to any other group (19·1±11·6, P=0·01).
Comparison of the TB and NTM groups revealed a tendency for the TB patients to have higher mean CD3 and CD8 percentages (73·9±15·5, 59·3±14·6) than the NTM participants (66·2±14·8, P=0·09; 52·3±10·9, P=0·07).
Immune profile according to CD4 cell counts
For the following analyses, participants were dichotomized according to CD4 counts above and below 200 cells. Of interest, significant differences in percentages of T and B lymphocytes existed within the same respiratory disease, according to CD4 cell counts, and are summarized in Table 2. In brief, patients with CAP and <200 CD4 counts exhibited higher NK and CD8 percentages (NK 14·3±10%, CD8 57·6±15·2%) than patients with >200 CD4 cells (9·3±5·7, P=0·001; 48·8 ±14·8, P=0·006).
Table 2.
Lymphocyte responses in patients’ respiratory diseases when dichotomized above and below 200 CD4 cell counts
Although not statistically significant, the NTM patients with <200 cells exhibited higher NK percentages (19·8±11·7%) than those with >200 counts (12±9·9%). TB patients with >200 CD4 cells showed higher CD8 percentages (61±14·5%) than TB patients with <200 cells (58·9±14·9, P=0·02).
Table 2 illustrates that in patients with PCP, B-cell percentages were lower in those with >200 CD4 cells (10·3±4·8), compared to their immunosuppressed counterparts (21±17·4, P=0·009). NK percentages were also significantly higher in AIDS patients (<200 CD4 counts) than in those with >200 CD4 counts (P=0·02). Although B-cell percentages were highest in the PCP group, followed by the CAP group, when patients with >200 CD4 counts were considered, the CAP group exhibited the highest B-cell percentages (P=0·009).
Respiratory infections, immune responses and HAART
A significantly lower proportion of the respiratory cases occurred in patients receiving HAART relative to those not using antiretroviral treatment. This is illustrated in 30% non-HAART treated PCP (P=0·005), 37% of the CAP (P=0·0004) and 45% of the mycobacterial diseases. HAART-treated patients accounted for 19% of the total admissions and more than one third (37% or 98 cases) of the respiratory group.
When compared to HAART-treated patients, those who did not receive HAART exhibited significantly lower CD4 values for each of the respiratory diseases (CAP, TB, NTM). Within the PCP group, HAART-treated patients tended to have higher NK cells (21·5 ±11·3) than non-HAART patients (15·4±8·6, P=0·09).
DISCUSSION
This pioneering study census compares, at hospital admission, the immunophenotype profiles of different respiratory diseases that are primary causes of HIV-related morbidity [1, 3, 9]. Understanding non-CD4+ T cell-dependent host defence mechanisms operative in infections represents an important contribution of this paper to those previously published [10–15]. These findings may have important implications for pathogenesis, diagnosis, and production of novel therapeutic approaches.
Although the function of macrophages and reactive oxygen metabolites has been thoroughly investigated, T and B lymphocyte responses are poorly understood. T and B cells regulate other immune cells inducing effector functions, as well as enhance antigen uptake, antibody production (B cells), and antigen degradation (CD8 and NK cells) [16]. We have also extended previous data by comparing immune responses between patients having CD4+ cells above and below 200 counts and evaluating the effects of HAART.
In accord with others, an inverse correlation of PCP and NTM with total CD4+ lymphocytes was observed [17–20]. Nonetheless, the immune profile of PCP patients differs from that of NTM subjects. The PCP group, for example, exhibited the highest B percentages, suggesting that humoral responses may represent an important clearance mechanism, particularly in those severely immunosuppressed (<200 CD4 cells). This is in agreement with animal models indicating that B-deficient mice with normal T cells are susceptible to P. jiroveci, and that the overall response to P. jiroveci is Th2 dominant, thus priming B-cell responses [21]. These data strengthen the suggestion that B cells are pivotal interaction partners for CD4+ T cells in the clearance of P. jiroveci and, therefore, B-cell modulation remains a valid approach to vaccine development.
The findings of low circulating CD8 percentages in the PCP group, further contribute to current knowledge as the roles of CD8 cells in host defence against PCP are far less clear, and animal models provided current information [19, 22]. Systemic depletion of CD8 cells has been associated with poor PCP clearance in rodents [19]. Since clearance of PCP is mediated by IFN-γ, produced by CD8 and NK cells, the increase of NK cells observed in our PCP patients could represent a compensatory mechanism [22]. Together, these findings indicate a role for both humoral and cellular responses in PCP host defence. These results also suggest that a humoral response is insufficient in patients with <200 CD4 cells, thus requiring NK-mediated clearance of PCP. The reasons for these differences and whether they are the consequence or cause of the infection cannot be determined from this investigation and merit further study.
In agreement with knockout models showing no CD8 impact on risk or outcome of NTM disease, our NTM patients had unaltered CD8 percentages [23]. However, these patients exhibited a dramatic increase in NK cells suggesting that NK cells may be more relevant in defence against NTM. Supporting this proposition, animals with functional defects or antibody-induced NK-cell depletion are more susceptible to disseminated Mycobacterium avium complex (MAC) [24].
Although non-tuberculous mycobacteria share significant structural and biochemical similarities with M. tuberculosis, they appear to induce different host-immune responses. In accord with others, our findings indicate elevated CD8+ T-cell percentages in TB/AIDS patients, compared to non-respiratory/HIV+ controls and HIV/AIDS patients with other respiratory infections [25]. Animal models support the conclusion that CD8+ T cells play an essential role in TB disease [26]. Our results also demonstrate that TB patients exhibit the lowest NK percentages across the patient groups. NK data are controversial; Nirmala et al. suggested that low NK percentages and activity during TB infection are probably the effect and not the cause of TB disease [27] while others reported no significant changes in NK cells between TB patients and controls [28]. Other studies have described an important role for NK cells in TB response [29] and even a detrimental role in late phases of mycobacterial infection [30] indicating the need for further research.
Enhanced understanding of susceptibility to and immune responses towards pneumococcal pneumonia is urgently needed, especially in the present HAART era when CAP is the most frequent cause of hospitalization. Our findings, in accord with others, indicate that CAP can be identified in HIV(+) patients, regardless of CD4 status or treatment [1, 3]. Moreover, modification in immune responses directed towards CAP failed to be detected in relation to antiretrovirals, suggesting the limited impact of HAART against CAP.
Factors, other than CD4, may alter the balance of the host immune response in favour of the microbe. CAP patients with >200 CD4 cells exhibited a distinct humoral action against bacteria, in contrast to the more immunosuppressed patients who showed a strong CD8 and NK cell response. Greater comprehension of the key events that control these interactions is needed.
While these findings are limited to those hospitalized at Jackson, UM/JMH represents one of the largest centres nationwide and the sample size is a census of all HIV-infected individuals hospitalized during the length of the study. Thus, similar results may be seen across the nation. Despite the limitations inherent to obtaining data in acutely ill patients, our findings are in accord with animal models, allowing us to postulate that most of the abnormalities observed probably lead these individuals to develop the respiratory disease. Nevertheless future longitudinal studies, using both immunophenotypic and functional immune evaluations, will be needed to confirm these findings. Increased understanding of the interaction between the immune system and different pathogens may indicate which immune effector mechanisms should be elicited by candidate immunogens for disease control.
ACKNOWLEDGEMENTS
This study was supported by the Florida Health Department [grant no. BM023 (M.J.M.B.)] and the Fogarty Training Program [grant no. 2D43TW00017 (G. S. P.)]. Gail Shor-Posner is the Director of the Division of Disease Prevention. Thanks are due to Jimmey Jackson for editorial support.
DECLARATION OF INTEREST
None.
REFERENCES
- 1.Wolff AJ, O’Donnell AE. Pulmonary manifestations of HIV infection in the era of highly active antiretroviral therapy. Chest. 2001;120:1888–1893. doi: 10.1378/chest.120.6.1888. [DOI] [PubMed] [Google Scholar]
- 2.Kim B, Lyons TM, Parada JP et al. HIV-related Pneumocystis carinii pneumonia in older patients hospitalized in the early HAART era. J Gen Intern Med. 2001;16:583–589. doi: 10.1046/j.1525-1497.2001.016009583.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Paul S, Gilbert HM, Zieheck W, Jacobs J, Sepkowitz KE. The impact of potent antiretroviral therapy on the characteristics of hospitalized patients with HIV infection. AIDS. 1999;13:415–418. doi: 10.1097/00002030-199902250-00015. [DOI] [PubMed] [Google Scholar]
- 4.Weissman D, Montaner LJ. Immune reconstitution. Clin Lab Med. 2002;22:719–740. doi: 10.1016/s0272-2712(02)00012-4. [DOI] [PubMed] [Google Scholar]
- 5.Valdez H. Immune restoration after treatment of HIV-1 infection with highly active antiretroviral therapy (HAART) AIDS Rev. 2002;4:157–164. [PubMed] [Google Scholar]
- 6.Centers for Disease Control. 1993 Revised Classification System for HIV Infection and Expanded Surveillance Case Definition for AIDS Among Adolescents and Adults. Morb Mortal Wkly Rep. 1992;41 RR-17. [PubMed] [Google Scholar]
- 7.Niederman MS, Mandell LA, Anzueto A et al. Guidelines for the management of adults with community-acquired pneumonia. Diagnosis, assessment of severity, antimicrobial therapy, and prevention. Am J Respir Crit Care Med. 2001;163:1730–1754. doi: 10.1164/ajrccm.163.7.at1010. [DOI] [PubMed] [Google Scholar]
- 8.Hirschtick RE, Glassroth J, Jordan MC, Wilcosky TC, Wallace JM. Bacterial pneumonia in persons infected with the human inmunodeficiency virus. N Engl J Med. 1995;333:845–851. doi: 10.1056/NEJM199509283331305. [DOI] [PubMed] [Google Scholar]
- 9.Zeller V, Caumes E, Bossi P, Bricaire F, Katlama C. Current clinical aspects of HIV/AIDS. Presse Med. 2002;31:74–79. [PubMed] [Google Scholar]
- 10.Caiaffa WT, Vlahov D, Graham NM et al. Drug smoking, Pneumocystis carinii pneumonia, and immunosuppression increase risk of bacterial pneumonia in human immunodeficiency virus-seropositive injection drug users. Amer J Resp Crit Car Med. 1994;150:1493–1498. doi: 10.1164/ajrccm.150.6.7952605. [DOI] [PubMed] [Google Scholar]
- 11.Forte M, Maartens G, Campbell F et al. T-lymphocyte responses to Pneumocystis carinii in healthy and HIV-positive individuals. J Acquir Immune Defic Syndr. 1992;5:409–416. [PubMed] [Google Scholar]
- 12.Bofill M, Lipman M, McLaughlin JE, Johnson MA, Poulter LW. Changes in lung lymphocyte populations reflect those seen in peripheral blood in HIV-1 positive individuals. Eur Respir J. 1998;11:548–553. [PubMed] [Google Scholar]
- 13.Hotz P, Loos U, Luderitz B. Lymphocyte subpopulations in bronchoalveolar lavage fluid in AIDS. Dtsch Med Wochenschr. 1994;119:289–295. doi: 10.1055/s-2008-1058693. [DOI] [PubMed] [Google Scholar]
- 14.Pitchenik AE, Fertel D, Bloch AB. Mycobacterial disease: epidemiology, diagnosis, treatment, and prevention. Clin Chest Med. 1988;9:425–441. [PubMed] [Google Scholar]
- 15.Pitchenik AE, Fertel D. Medical management of AIDS patients. Tuberculosis and nontuberculosis mycobacterial disease. Med Clin North Am. 1992;76:121–171. doi: 10.1016/s0025-7125(16)30375-3. [DOI] [PubMed] [Google Scholar]
- 16.Beck JM, Harmsen AG. Lymphocytes in host defense against Pneumocystis carinii. Semin Respir Infect. 1988;13:330–338. [PubMed] [Google Scholar]
- 17.Stansell JD, Osmond DH, Charlebois E et al. Predictors of Pneumocystis carinii pneumonia in HIV-infected persons. Pulmonary Complications of HIV Infection Study Group. Am J Respir Crit Care Med. 1997;155:60–66. doi: 10.1164/ajrccm.155.1.9001290. [DOI] [PubMed] [Google Scholar]
- 18.Field SK, Fisher D, Cowie RL. Mycobacterium avium complex pulmonary disease in patients without HIV infection. Chest. 2004;126:566–581. doi: 10.1378/chest.126.2.566. [DOI] [PubMed] [Google Scholar]
- 19.Steele C, Zheng M, Young E, Marrero L, Shellito JE, Kolls JK. Increased host resistance against Pneumocystis carinii pneumonia in gammadelta T-cell-deficient mice: protective role of gamma interferon and CD8(+) T cells. Infect Immun. 2002;70:5208–5215. doi: 10.1128/IAI.70.9.5208-5215.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Benator DA, Gordin FM. Notuberculous mycobacteria in patients with human immunodeficiency virus infection. Semin Respir Infect. 1996;11:285–300. [PubMed] [Google Scholar]
- 21.Hanano R, Kaufmann SH. Pneumocystis carinii pneumonia in mutant mice deficient in both TCRalphabeta and TCRgammadelta cells: cytokine and antibody responses. J Infect Dis. 1999;179:455–459. doi: 10.1086/314607. [DOI] [PubMed] [Google Scholar]
- 22.Kolls JK, Habetz S, Shean MK et al. IFN-gamma and CD8+ T cells restore host defenses against Pneumocystis carinii in mice depleted of CD4+ T cells. J Immunol. 1999;162:2890–2894. [PubMed] [Google Scholar]
- 23.Bermudez LE, Petrofsky M. Host defense against Mycobacterium avium does not have an absolute requirement for major histocompatibility complex class I-restricted T cells. Infect Immun. 1999;67:3108–3111. doi: 10.1128/iai.67.6.3108-3111.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gangadharam PR. Beige mouse model for Mycobacterium avium complex disease. Antimicrobial Agents Chemother. 1995;39:1647–1654. doi: 10.1128/aac.39.8.1647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hohn H, Julch M, Pilch H et al. Definition of the HLA-A2 restricted peptides recognized by human CD8+ effector T cells by flow-assisted sorting of the CD8+ CD45RA+CD28-T cell subpopulation. Clin Exp Immunol. 2003;131:102–110. doi: 10.1046/j.1365-2249.2003.02036.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lazarevic V, Flynn J. CD8+ T cells in tuberculosis. Am J Res Crit Care Med. 2002;166:1116–1121. doi: 10.1164/rccm.2204027. [DOI] [PubMed] [Google Scholar]
- 27.Nirmala R, Narayanan PR, Mathew R, Maran M, Deivanayagam CN. Reduced NK activity in pulmonary tuberculosis patients with/without HIV infection: identifying the defective stage and studying the effect of interleukins on NK activity. Tuberculosis. 2001;81:343–352. doi: 10.1054/tube.2001.0309. [DOI] [PubMed] [Google Scholar]
- 28.Montes Santiago J, Gambon Deza F, Pacheco Carracedo M, Cerda Mota T. Cellular immune response in tuberculosis: analysis of T-lymphocytes and their subsets, B-lymphocytes and natural cytotoxic cells in different tuberculosis states and body fluids. Revista Clinica Espanola. 1996;196:223–227. [PubMed] [Google Scholar]
- 29.Saunders BM, Frank AA, Orme IM, Cooper AM. CD4 is required for the development of a protective granulomatous response to pulmonary tuberculosis. Cell Immunol. 2002;216:65–72. doi: 10.1016/s0008-8749(02)00510-5. [DOI] [PubMed] [Google Scholar]
- 30.Sugawara I, Yamada H, Mizuno S, Li CY, Nakayama T, Taniguchi M. Mycobacterial infection in natural killer T cell knockout mice. Tuberculosis. 2002;82:97–104. doi: 10.1054/tube.2002.0331. [DOI] [PubMed] [Google Scholar]