SUMMARY
The study sought to identify factors involved in the emergence, prevention and elimination of severe acute respiratory syndrome (SARS) in Hong Kong during 11 March to 22 May 2003. A structured multiphase regression analysis was used to estimate the potential effects of weather, time and interaction effect of hospital infection. In days with a lower air temperature during the epidemic, the risk of increased daily incidence of SARS was 18·18-fold (95% confidence interval 5·6–58·8) higher than in days with a higher temperature. The total daily new cases might naturally decrease by an average of 2·8 patients for every 10 days during the epidemic. The multiplicative effect of infected hospital staff with patients in an intensive care unit (ICU) and the proportion of SARS patients in ICUs might respectively increase the risk of a larger SARS epidemic in the community. The provision of protective gear in hospitals was also a very important factor for the prevention of SARS infection. SARS transmission appeared to be dependent on seasonal temperature changes and the multiplicative effect of hospital infection. SARS also appeared to retreat naturally over time.
INTRODUCTION
Severe acute respiratory syndrome (SARS) caused by a novel coronavirus is a recently described illness in humans [1–4]. It was named, in 2003, after the large-scale outbreaks that occurred in Mainland China, Hong Kong, Taiwan, Canada and Singapore [5–8]. In Hong Kong, the outbreak during 11 March to 22 May 2003, resulted in a total of 1755 patients with SARS and 298 deaths.
In order to understand the epidemic and be prepared for any future return of SARS, efforts have been made to examine the factors that influenced its spread. Effectiveness of some interventions against SARS, epidemiological determinants and gender have previously been described [9–14]. However, other factors remain to be examined for their potential effects on the emergence, prevention and elimination of SARS. In particular, there has been no study on whether and how weather and hospital infection affected the transmission of SARS. Incidentally, the SARS outbreak in Hong Kong occurred in winter (March) and gradually died out as spring came. Therefore, it may well be the case that the SARS virus might share a similar seasonal behaviour as that of the influenza virus. On the other hand, the SARS outbreak in Hong Kong started from a hospital ward. Therefore, hospital infection including the number of SARS patients under intensive care and the number of infected hospital staff may possibly be important sources of SARS transmission.
By collecting the daily numbers of newly confirmed SARS patients in Hong Kong during the outbreak, this study aimed to identify potential factors involved in the emergence, prevention and elimination of SARS.
Methods
Data collection
Daily number of SARS patients
The daily number of newly confirmed SARS patients in Hong Kong during the outbreak between 11 March and 22 May, 2003 was obtained from an integrated database, coordinated by one of the authors (J.K.) from the Clinical Trials Centre at the Faculty of Medicine of The University of Hong Kong. On 23 May 2003, the SARS outbreak in Hong Kong was over, as the World Health Organization (WHO) cancelled the recommendation against unnecessary travel to Hong Kong. The diagnosis of SARS was based on clinical signs, chest X-ray, diagnostic tests in some patients and/or autopsy [10]. The daily number of newly confirmed SARS patients was also broken down into hospital staff, residents of Amoy Gardens, and the community at large excluding Amoy Gardens. Moreover, SARS figures were also obtained concerning daily hospital discharges, deaths and patients in intensive care units (ICUs).
Interventional measures
Three interventions against the SARS outbreak were considered: (1) Since 17 March 2003, a total of 13 interventions were made [10]; (2) on 30 March 2003, residents of Block E, Amoy Gardens were quarantined for 10 days [15]; and (3) since 11 April 2003, the Hong Kong Hospital Authority provided protective gear for frontline staff in hospitals.
Daily meteorological data
Daily meteorological data between 11 March and 22 May 2003 were obtained from the Hong Kong Observatory. The daily data available included mean air temperature (°C), mean atmospheric relative humidity (%), mean atmospheric pressure (hPa), and temperature difference (i.e. maximum–minimum).
Hospital infection and its multiplicative effect
These factors included: (1) the daily number of SARS patients in ICUs, (2) the proportion of SARS patients in ICUs (i.e. number of SARS patients in ICUs/cumulative number of SARS patients), and (3) the multiplicative effect of the number of SARS patients in ICUs (NICU) and the daily number of new hospital staff cases (DNHSC) of hospital infection [i.e. (NICU+1)×(DNHSC+1)].
Study design and data analyses
The study sought to identify the factors and estimate the effects of the factors involved in SARS emergence, prevention and elimination. These factors would be related to the daily incidences of SARS cases, i.e. the total number of SARS patients in Hong Kong. It was also speculated that the factors were related to the breakdown of SARS cases in hospital staff, residents of Amoy Gardens, and the community at large. The mean incubation period was reported to be 6·4 days [10]. Therefore, the daily numbers of newly infected cases (defined as the number of new infections occurring in a single day leading to manifestation of a full-blown case) were lagging behind the daily numbers of newly confirmed cases by ∼6 days.
Continuous factors were first categorized by the corresponding median values. The effect of each factor on the daily incidences of SARS was then examined individually. A structured multiphase regression analysis was performed on three hierarchical clusters of variables with a hypothesized causal relationship [16]. The first cluster included all the daily meteorological conditions. The second cluster was comprised of the three interventional measures. The third cluster contained (1) the epidemic day (i.e. the number of days since the epidemic began in Hong Kong on 11 March 2003), (2) hospital infection sources, (3) the interaction between hospital infection sources, and (4) the daily numbers of newly confirmed patients. We hypothesized that a variable in Cluster 1 may influence variables in Cluster 2 but not vice versa. The same relationship also holds for Clusters 2 and 3. As a result, simultaneous consideration of variables from all clusters in a conventional stepwise multiple regression analysis might result in confounded inference of the effects. Instead, a regression analysis was conducted in three phases for each of the four daily numbers of new infections (total, hospital staff, Amoy Gardens residents and community at large excluding Amoy Gardens), i.e. the daily number of newly confirmed SARS cases put forward by 6 days. Phase 1 was a forward stepwise regression on Cluster 1 variables. Phase 2 introduced into the model all variables selected in Phase 1 before Cluster 2 variables were entered by the same forward stepwise approach. Similar steps were performed for Clusters 2 and 3 variables. In addition, a structured multiphase logistic regression analysis was also performed to estimate the risk [i.e. odds ratio (OR)] of the occurrence of a larger SARS epidemic. As such, a larger SARS epidemic was deemed to have occurred when the daily incidence of SARS was larger than its median value.
Model assumptions at each phase of the analysis were examined by using the deleted studentized residuals after collinearity diagnostics for independent variables were made. An unpaired t test was performed to compare averages. All significance tests were two-tailed and a P value of <0·05 was taken as being statistically significant. Statistical analysis was performed in SPSS 10.0 for Windows (SPSS Inc., Chicago, IL, USA).
Results
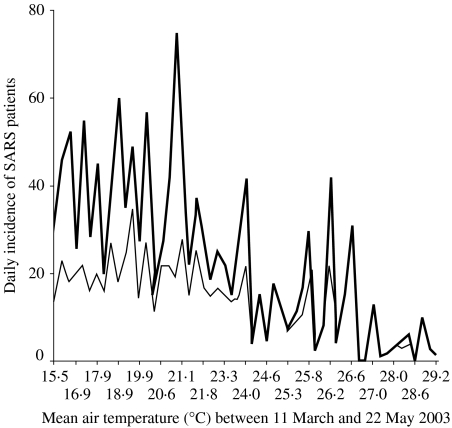
Table 1 shows the factors associated with the daily incidence of SARS when they were analysed individually. In particular, the relationship between the daily numbers of SARS patients and the daily mean air temperature is depicted in the Figure. Tables 2 and 3 summarize results from the structured multiphase regression analysis for the daily evolution of SARS cases and the occurrence of a larger SARS epidemic.
Table 1.
Factors associated with daily incidence of SARS in Hong Kong when analysed individually
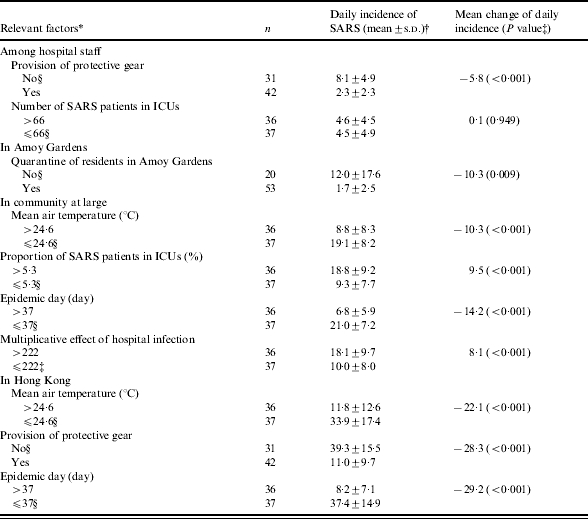
Except for provision of protective gear, all other factors were categorized by their respective medians.
s.d., Standard deviation.
By Student’s t test.
Reference level.
Fig.
Relationship between the daily number of SARS patients and the daily mean air temperature in Hong Kong during 11 March to 22 May 2003. , Total;
, Total;  , community.
, community.
Table 2.
Factors related to the daily incidence of SARS in Hong Kong between 11 March and 22 May 2003*
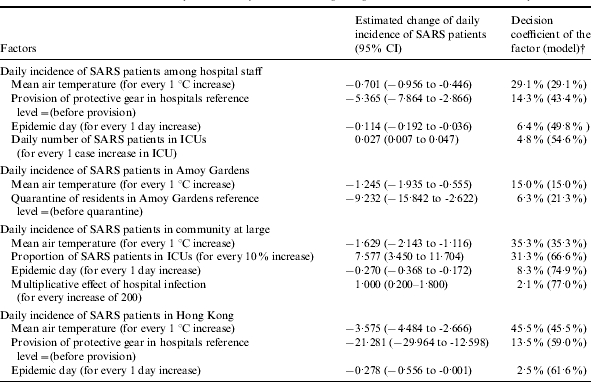
Structured multiphase regression analysis was performed.
Decision coefficient of factor=partial R2 for the factor; decision coefficient of model=model R2.
Table 3.
Risk factors and prevention measures related to the risk of a larger SARS epidemic occurring in Hong Kong between 11 March and 22 May 2003*
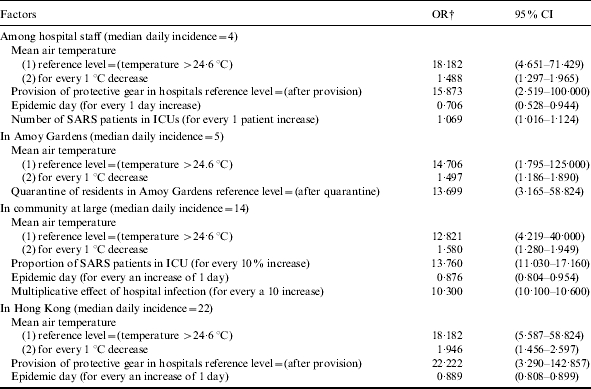
OR, Odds ratio; CI, confidence interval.
Structured multiphase logistic regression analysis was performed to estimate odds ratios. Odds ratio measures the increase in risk of a daily incidence of SARS incidence more than its median.
All of them were significant with a P value <0·001.
Factors of SARS spread among hospital staff
The daily mean air temperature was the only significant meteorological factor. An increase of 1°C in air temperature was related to an average reduction of 0·7 staff patients. The provision of protective gear for hospital staff was the only significant interventional measure, associated with an average reduction of 5·4 daily new staff patients (Table 2). Before the provision of protective gear, the risk of a larger SARS epidemic, i.e. having the daily incidence of hospital staff patients larger than the median of four, was 15·87-fold higher [95% confidence interval (CI) 2·52–100·00, P<0·001]. Further, the risk of a larger SARS epidemic may increase by 1·07-fold for every increase of one ICU case (95% CI 1·02–1·12, P<0·001) (Table 3). Therefore, the daily number of SARS patients in ICUs may be an important factor in the emergence of SARS among hospital staff.
Factors of SARS spread in Amoy Gardens
The daily mean air temperature remained as the sole significant meteorological factor. An increase of 1°C in the air temperature was associated with an average reduction of 1·2 patients in Amoy Gardens. The quarantine measure of residents of Block E in Amoy Gardens was the only significant interventional measure, correlated with an average reduction of 9·2 daily new cases (Table 2). Before the implementation of the quarantine measure, the risk of a larger SARS epidemic was 13·70-fold higher (Table 3).
Factors of SARS spread in the community at large
Among daily new cases in the community at large excluding Amoy Gardens, the daily mean air temperature, epidemic day, multiplicative effect of hospital infection, and the proportion of patients in ICUs were significant factors (Tables 2 and 3). During the epidemic in days with lower air temperature the risk of a larger SARS epidemic in the community was 12·82-fold (95% CI 4·22–40·00, P<0·001) higher than that in days with higher air temperature (i.e. a temperature >24·6°C) (Table 3). An increase of 1°C in air temperature was associated with an average reduction of 1·6 patients. The epidemic day and multiplicative effect of hospital infection respectively explained 8% and 2% of variance for the daily number of increased cases in the community (Table 2). The risk of a larger SARS epidemic may increase by 10·30-fold for every 10-unit increase in the multiplicative effect of hospital infection (95% CI 10·10–10·60, P<0·001). After accounting for the effect of air temperature, the OR of a larger epidemic for a 10% increase in the proportion of patients in ICUs was 13·76 (95% CI 11·03–17·16, P<0·001) (Table 3). A 10% increase in the proportion of patients in ICUs was related to the introduction of 7·6 additional cases per day. The multiplicative effect of hospital infection and the proportion of patients in ICUs may be important factors involved in the spread of SARS in the community. These factors explained 77% of the total variance for the daily number of increased SARS patients in the community (Table 2). No interventional measures during the epidemic were found to be significant.
Factors of SARS spread in Hong Kong
During the epidemic in days with lower air temperature the risk of a larger SARS epidemic with the daily SARS incidence larger than the median (22 patients) was 18·18-fold (95% CI 5·59–58·82, P<0·001) higher than that in days with higher temperature (Table 3). An increase of 1°C in air temperature was associated with an average reduction of 3·6 cases. Air temperature might be a very important factor in the emergence and elimination of SARS. Moreover, the total daily new cases decreased by an average of 2·8 cases for every 10 epidemic days, even after accounting for the effects of weather and the various interventional measures. The provision of protective gear to hospital staff was the only significant interventional measure which had a correlation with an average reduction of 21·3 daily new cases (Table 2). Before the provision of protective gear, the risk of a larger SARS epidemic in Hong Kong was 22·22-fold higher (Table 3). The three factors together explained 62% of the total observed variance for Hong Kong’s daily cases (Table 2).
DISCUSSION
Understanding of the factors involved in the emergence, prevention and elimination of SARS will be of great value in the control of future SARS spread. Answers to whether the SARS outbreak is seasonal may help to plan for the proper management of SARS. The results of this study suggest that the mean air temperature, epidemic day, control of SARS hospital infection, hospital infectious sources and their multiplicative effect, together with the quarantine measure in Amoy Gardens were associated with SARS transmission and the control of it. The provision of protective gear for hospital staff and the quarantine in Amoy Gardens appeared to be successful in controlling the spread of SARS in Hong Kong. In particular, we found that lower air temperature and the multiplicative effect of hospital infection might respectively increase the risk of a larger SARS epidemic. In addition, the SARS epidemic might naturally retreat over time.
In general, transmissible agent, host and environment are the three factors that affect the epidemiology of communicable diseases. Since respiratory diseases are more common in late winter and early spring, the occurrence of SARS might, in part, be determined by environmental factors. An ecological study has demonstrated a positive association between air pollution and SARS case fatality in the Chinese population [17]. Our results showed, for the first time, that under lower air temperature, the number of incidences of SARS appeared to be increased. The number of incidences under lower air temperature was 18·18-fold higher than that under higher temperature. The SARS viruses would possibly disappear gradually along with the temperate increase of air temperature in the course of the epidemic in Hong Kong. Hence, air temperature might be a very important factor in the emergence of SARS and its elimination. This might be due to a decrease in resistance to diseases of the respiratory system in a colder environment. In addition, as the outdoor temperature decreases, a warm indoor environment may increase the infection rate if ventilation is poor. In a warmer climate, the agent might not be able to withstand the environmental changes, resulting in the decay of its virulence. Hence, daily SARS cases in Hong Kong might decline gradually with an increase in air temperature and a change of other weather conditions.
However, the effect of air temperature may be confounded by other factors. The potential ‘come-and-go’ feature of the coronavirus – with fluctuations in air temperature – is similar to the influenza virus [18]. In fact, new SARS patients have emerged in China since January 2004 [19] – consistent with the potential effect of lower air temperature on the coronavirus. However, a longer history of the SARS epidemic is needed before proper epidemiological estimation of seasonal and meteorological effects on SARS can be made.
Our analysis also showed that the SARS epidemic might naturally retreat over time even after accounting for the effects of weather and the various interventional measures. The epidemic day may be another factor in the elimination of SARS. The occurrence of infection and its outcome are in part determined by host factors. We supposed that with the cumulative number of recovered patients, inapparent patients and death cases during the evolution of the epidemic, the number of persons who acquired immunity against SARS might have been increased. Therefore, infectious sources may gradually decrease in the population. Moreover, the virulence of the infectious agent might also decrease gradually in the epidemic. Hence, the SARS epidemic would be likely to abate gradually. The epidemic day might indirectly reflect the change of the number of susceptible persons, infection sources and the virulence of the infectious agent.
Based on the above findings, when the effect of any factor influencing SARS transmission is estimated, the confounding factors (i.e. weather and time) must first be considered because air temperature and epidemic day could confound the effects of the other factors.
On the other hand, we found that the number of SARS patients in ICUs was an important risk factor of SARS transmission among hospital staff. The greater the daily number of SARS patients in ICUs, the more severe was the SARS epidemic that occurred among hospital staff after a mean incubation period of 6 days. Therefore, patients in ICUs appeared to be an important source of hospital infection. Indeed, the SARS outbreak in Hong Kong started in the ward of a local hospital – while contact tracing indicated that >22% of cases in Hong Kong were associated with hospital-related exposure [13]. SARS patients among hospital staff and in ICUs together constituted the source of hospital infection. The results also showed that a greater proportion of patients in ICUs might increase the risk of a larger SARS epidemic in the community at large, excluding Amoy Gardens. We found that the interaction effect of infected hospital staff and patients in ICUs on potentially speeding-up the transmission of SARS in the community was a multiplicative effect and not a simple additive effect. The multiplicative effect of hospital infection also appeared to increase the risk of a greater SARS transmission in the community.
In view of the increased number of SARS cases among hospital staff, the Hong Kong Hospital Authority have provided protective gear to hospital staff since 11 April 2003 for effective infection control of the disease. The gear included powered air purification respirator hood, non-reusable goggles or face-shield, and protective gowns. Our results showed that this measure appeared to significantly decrease the number of incidences of SARS among hospital staff and even in the community. It possibly interrupted the spread pathway between SARS patients and hospital staff, consequently inhibiting the spread into the community through its multiplicative effect with the patients in ICUs. This provides a possible interpretation of the findings that SARS transmission could be controlled by measures used for the control of nosocomical infection. These results confirm our belief that stronger measures for controlling hospital infection are needed and hospital infection control is a key to controlling the transmission. The epidemic might be better controlled if hospital infection control was administered in the initial phase of the SARS epidemic. Furthermore, SARS is a contagious and rapidly progressive infectious disease. Droplets precautions, rigorous universal precautions or standard precautions were recommended for the care of patients with SARS. Infection control measures for health-care workers exposed to patients with SARS should include the measures of staff education, dress precautions, ICU environment and equipment, and transport and ventilation of the patients.
Between 26 March 2003 and 24 April 2003, a major point-source outbreak of SARS involving 329 patients occurred in the Amoy Gardens in Hong Kong. The epidemiological investigation suggested that the SARS outbreak in Amoy Gardens was caused by a faulty sewage system contaminated by an index patient [15]. In 2003 Ng proposed an alternative hypothesis that the source of rapid transmission of the causal agent might have been an animal vector, most probably roof rats [20]. Nevertheless, our results showed that the quarantine of residents of Amoy Gardens to rural quarantine camps appeared to significantly decrease incidences of SARS in the housing estate. Therefore, the measure might be highly effective in interrupting the pathway of transmission between potential origins of SARS infection to residents.
In contrast, no significant effect of the measure of multiple interventions was found on any of the four daily tallies of SARS cases. This indicates that they might be not as effective as other factors in epidemic control.
In summary, air temperature, epidemic day, the multiplicative effect of infected hospital staff and ICU patients, the proportion of cases in ICUs, provision of protective gear for hospital staff, and quarantine in Amoy Gardens were important factors associated with the emergence, prevention and elimination of SARS. These factors could explain about 62% of the variance for the daily total number of new SARS patients in Hong Kong. However, unknown factors accounted for the remaining 38% of variance. A potential viral animal reservoir in SARS may be a factor in transmission worthy for further study [21].
ACKNOWLEDGEMENTS
We gratefully acknowledge the help of the Hong Kong Observatory in supplying meteorological observations for Hong Kong. Funding was received from Department of Nursing Studies and the Clinical Trials Centre, Faculty of Medicine, the University of Hong Kong; Department of Science Technology of Guangdong Province (2004B33701013), and the Health Office of Guangdong Province (A2003485).
DECLARATION OF INTEREST
None
REFERENCES
- 1.World Health Organization. Severe acute respiratory syndrome (SARS) Wkly Epidemiol Rec. 2003;78:86. [Google Scholar]
- 2.Ksiazek TG, Erdman D, Goldsmith CS et al. A novel coronavirus associated with Severe Acute Respiratory Syndrome. N Engl J Med. 2003;348:1953–1966. doi: 10.1056/NEJMoa030781. [DOI] [PubMed] [Google Scholar]
- 3.Drosten C, Gunther S, Preiser W et al. Identification of a novel coronavirus in patients with Severe Acute Respiratory Syndrome. N Engl J Med. 2003;348:1967–1976. doi: 10.1056/NEJMoa030747. [DOI] [PubMed] [Google Scholar]
- 4.Peiris JS, Lai ST, Poon LL et al. Coronavirus as a possible cause of severe acute respiratory syndrome. Lancet. 2003;361:1319–1325. doi: 10.1016/S0140-6736(03)13077-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tsang KW, Ho PL, Ooi GC et al. A cluster of cases of severe acute respiratory syndrome in Hong Kong. N Engl J Med. 2003;348:1977–1985. doi: 10.1056/NEJMoa030666. [DOI] [PubMed] [Google Scholar]
- 6.Lee N, Hui D, Wu A et al. A major outbreak of severe acute respiratory syndrome in Hong Kong. N Engl J Med. 2003;348:1986–1994. doi: 10.1056/NEJMoa030685. [DOI] [PubMed] [Google Scholar]
- 7.World Health Organization. http://www.who.int/csr/sars/country/2003_07_11/en/ http://www.who.int/csr/sars/country/2003_07_11/en/ Cumulative number of reported cases (SARS) from Feb. 1, 2003, to July 11, 2003. ). Accessed 3 April 2004.
- 8.Welfare and Food Bureau of Hong Kong, Hong Kong Special Administrative Region. http://www.info.gov.hk/dh/diseases/ap/eng/bulletin.htm. http://www.info.gov.hk/dh/diseases/ap/eng/bulletin.htm SARS bulletin. ). Accessed 3 April 2004.
- 9.Dye C, Gay N. Epidemiology: Modeling the SARS epidemic. Science. 2003;300:1884–1885. doi: 10.1126/science.1086925. [DOI] [PubMed] [Google Scholar]
- 10.Donnelly CA, Ghani AC, Leung GM et al. Epidemiological determinants of spread of causal agent of severe acute respiratory syndrome in Hong Kong. Lancet. 2003;361:1761–1766. doi: 10.1016/S0140-6736(03)13410-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Karlberg J, Chong DSY, Lai WYY. Do men have a higher SARS case fatality risk than women. Am J Epidemiol. 2004;159:229–231. doi: 10.1093/aje/kwh056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Seto WH, Tsang D, Yung RWH et al. Effectiveness of precautions against droplets and contact in prevention of nosocomial transmission of severe acute respiratory syndrome (SARS) Lancet. 2003;361:1519–1520. doi: 10.1016/S0140-6736(03)13168-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rily S, Fraser C, Donnelly CA et al. Transmission dynamics of the etiological agent of SARS in Hong Kong: impact of public health interventions. Science. 2003;300:1861–1865. doi: 10.1126/science.1086478. [DOI] [PubMed] [Google Scholar]
- 14.Lipsitch M, Cohen T, Cooper B et al. Transmission dynamics and control of severe acute respiratory syndrome. Science. 2003;300:1966–1970. doi: 10.1126/science.1086616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Peiris JSM, Chu CM, Cheng VCC et al. Clinical progression and viral load in a community outbreak of coronavirus-associated SARS pneumonia: a prospective study. Lancet. 2003;361:1767–1772. doi: 10.1016/S0140-6736(03)13412-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lam CLK, Fong DYT, Lauder IJ, Lam TPD. The effect of health-related quality of life (HRQOL) on health service utilisation of a Chinese population. Soc Sci Med. 2002;55:1635–1646. doi: 10.1016/s0277-9536(01)00296-9. [DOI] [PubMed] [Google Scholar]
- 17.Cui Y, Zhang Z, Froines Z http://www.ehjournal.net/content/2/1/15. http://www.ehjournal.net/content/2/1/15 Air pollution and case fatality of SARS in the People’s Republic of China: an ecologic study. Environmental Health: A Global Access Science Source. 20 Nov. 2003. ). Accessed 3 April 2004. [DOI] [PMC free article] [PubMed]
- 18.Frost J. Influenza – the seasonal virus. Prof Nurse. 1991;6:242–244. [PubMed] [Google Scholar]
- 19.World Health Organization. http://www.wpro.who.int/sars/docs/pressreleases/pr_17012004.asp. http://www.wpro.who.int/sars/docs/pressreleases/pr_17012004.asp WHO update on the two suspect SARS cases in Guangdong Province. ). Accessed 3 April 2004.
- 20.Ng SKC. Possible role of an animal vector in the SARS outbreak at Amoy Gardens. Lancet. 2003;362:570–572. doi: 10.1016/S0140-6736(03)14121-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Guan Y, Zheng BZ, He YQ et al. Isolation and characterization of viruses related to the SARS coronavirus from animals in Southern China. Science. 2003;302:276–278. doi: 10.1126/science.1087139. [DOI] [PubMed] [Google Scholar]