SUMMARY
In January 2003, two cases of Legionnaires’ disease associated with a ship’s cruise were registered in the database of National Epidemiological Surveillance of Infectious Diseases. A 70-year-old male heavy smoker with mild emphysema contracted the disease during a cruise. Legionella pneumophila serogroup (sg) 5 was isolated from the patient’s sputum and the ship’s indoor spa. The isolate from the spa matched the patient’s isolate by genotyping performed by pulsed-field gel electrophoresis (PFGE). The second case was in a 73-year-old female. During epidemiological investigation, a third case of Legionnaire’s disease in a 71-year-old male was subsequently diagnosed among passengers on the same ship on the following cruise. Environmental investigation revealed that porous natural stones (Maifanshi) in the filters of the spas had harboured L. pneumophila, a phenomenon which has not been reported except in Japan. This is the first documented evidence of L. pneumophila sg 5 infection on a ship and of porous stones as a source of Legionella infection.
INTRODUCTION
Legionnaires’ disease is a severe form of atypical pneumonia often caused by L. pneumophila, which in the United States from 1980 to 1998 accounted for 91·4% of clinical isolates [1]. Of the L. pneumophila isolates whose serogroup (sg) was known, 85% were sg 1, and only 1·9% were sg 5. In Japan from 1999 to 2002, 91% of clinical isolates for Legionnaires’ disease were L. pneumophila; and among known serogroups, 65% and 3·8% were L. pneumophila sg 1 and sg 5 respectively [2]. Similarly, most ship-associated cases have been ascribed to infection by L. pneumophila sg 1, and isolation of sg 5 has been rare [3], only one being previously reported, in a Pacific cruise out of Sydney [4]. A molecular link between the disease and a source of infection on a ship has been documented in only two clusters caused by L. pneumophila sg 1 [5, 6].
In this report, we describe an outbreak of Legionnaires’ disease caused by L. pneumophila sg 5 which was molecular-epidemiologically linked to the spa baths during the two cruises on the same ship.
MATERIALS AND METHODS
Description of the cases
Case 1 (the index case in this cluster)
A 70-year-old Japanese man had been a passenger on a cruise ship with his wife from 27 December 2002 to 5 January 2003. They had never stayed ashore overnight. He had used the men’s indoor spa bath at least seven times during the cruise. He presented with dry cough and mild fever since 1 January and with diminished appetite and general fatigue since 6 January [7]. On 13 January, he was admitted to the emergency room with severe dyspnoea. L. pneumophila sg 5 was isolated from the patient’s sputum collected on the day of admittance, and urinary antigen was detected by Binax NOW Legionella immunochromatographic test (Binax Inc., Portland, ME, USA) in a specimen collected on hospital day 3. He rapidly developed multiple organ failure accompanied by septicaemia. Although the patient was discharged from the intensive care unit on day 22 of his hospitalization, the disease resulted in chronic need for supplemental oxygen.
Case 2
A 73-year-old female passenger on the same cruise ship had complained of cough and fever since 3 January. On 14 January, she was hospitalized for dyspnoea and severe pneumonia [8]. Biotest EIA test (Biotest AG, Dreieich, Germany) and Binax NOW Legionella immunochromatographic test detected urinary antigen of ‘serogroups 1 and/or 5’. Culture and PCR of a sputum specimen were negative. By microplate agglutination test, single-serum antibody titres against L. pneumophila sg 1 to sg 6 were also negative. In spite of macrolide and aminoglycoside antibiotic therapy and steroid pulse therapy, she developed a pulmonary thromboembolism but on 16 March 2003 was finally discharged from the hospital. She had used the women’s indoor spa bath in the cruise. Her home bath was not a spa, and the bath water was emptied after use every day. She had used neither pool nor spa except for the women’s indoor spa and possibly also the whirlpool spa on the ship. There was no fountain in the public gardens near her home. No legionella was isolated from the female patient’s home bath.
Case 3
A 71-year-old male passenger on the subsequent cruise from 7 to 9 January 2003, had presented with cough since 11 January and with fever of 38–39 °C since 12 January. He consulted a physician and was diagnosed without roentgenography as having bronchitis [9]. He had used the men’s indoor spa bath twice a day for 3 days, but neither a whirlpool spa nor a warm pool during the cruise. After the epidemiological investigation had begun, he was informed of the occurrence of ship-associated Legionnaires’ disease and visited the local community hospital on 30 January. By Biotest EIA test, his urinary antigen of probable ‘serogroup 5’ was positive and he was diagnosed as having Legionnaires’ disease and was hospitalized on the same day. Sputum culture and paired sera test were both negative. Pathological examination revealed bronchitis and interstitial pneumonia. He was discharged on 15 February. His home bath was not a spa and he had not travelled except for the cruise.
Infection of other passengers
The cruise ship was a 26500-ton Japanese passenger ship with capacity for 696 passengers and 204 crew members. The first cruise of the ship was in 1998. There were 238 passenger rooms on the ship, of which 40 rooms had baths and the others showers. On 28 January 2003, based on one confirmed case and a probable source of infection in the cruise ship’s environment, 1821 of the 1833 passengers who had travelled in a total of five separate cruises on the same ship from 27 December 2002 to 19 January 2003 were informed of the occurrence of Legionnaires’ disease associated with the ship. All were advised to consult a physician in case of illness. This resulted in the finding of case 3, and an additional 18 passengers with illness (two with pneumonia, and the other 16 with fever, cough, nausea, and/or diarrhoea) after the cruises. However, the two other pneumonia patients were diagnosed as not having Legionnaires’ disease, and no further case was found. The numbers of passengers on the first and second cruises, where confirmed cases were found, were 459 and 514 respectively. About 200 passengers enjoyed the indoor spas every day and the male:female ratio of indoor spa users was 3:2. Users of the whirlpool spa on the deck were fewer than 10 a day.
Environmental sampling
On 23 December 2002, three water samples collected from spas and one cabin’s bath on the ship were tested by a private inspection company. These specimens were obtained before any remedial decontamination. Further water samples collected from the spas on 14 January 2003 were tested at a legally incorporated foundation. All environmental samples mentioned above had been voluntarily collected by the ship’s company for Legionella tests before the confirmation of the first patient. On 28 January 2003, when the outbreak was first suspected, a public health centre in Hyogo prefecture, where the ship was docked, performed environmental investigations. Sterile swabs were dipped in sterile saline, rotated against a 10-cm2 surface area, kept and transported in a tube containing 10 ml of saline. Three residual water samples and 12 swabs were collected by public health officers at different sites on the ship, especially the spas. Two samples of porous natural stones (Maifanshi) used as filter bed material in the indoor-spa filters were collected on the same day, immersed and transported in sterile water. These stones and their immersion water were tested separately. Maifanshi is also called China medical stone. It is found in Inner Mongolia and in Japan, and is a kind of intrusion of granodiorite porphyry composed of aluminosilicates [10]. In recent years, Maifanshi have been used in Japan for producing artificial mineral water and as a refrigerator odour killer.
Laboratory methods
Water samples and swab suspensions were concentrated by filtration (0·22 μm pore size). The stones were put into an airtight container and sonicated in saline at 130 W and 20 kHz for 24 s to collect residual biofilms from inside the stones. The turbid extract was concentrated by centrifugation. Diluted and undiluted specimens were plated on buffered charcoal yeast extract (BCYE) α-non-selective and WYO α-selective agars (Eiken Chemical, Tokyo, Japan) [11], with and without heat or acid treatment, following the usual procedures for the isolation of Legionella spp. Identification of Legionella isolates was performed using species-specific and serogroup-specific monovalent antisera (Denka Seiken, Tokyo, Japan). Some isolates were confirmed by PCR with specific primers for Legionella 5S rRNA DNA and the L. pneumophila mip gene [primers were prepared according to the sequences from the formerly commercially available EnviroAmp Legionella (PerkinElmer Cetus Corporation, Norwalk, CT, USA)]. All available Legionella strains isolated from samples were sent to the Legionella Reference Centre, Department of Bacteriology in the National Institute of Infectious Diseases in Japan. To show the link between clinical and environmental L. pneumophila sg 5 isolates, strains were analysed by use of PFGE of genomic DNA digested with SfiI restriction enzymes [12]. Unfortunately, the isolates from the samples tested at the private inspection company were discarded after testing, which made retests impossible.
To isolate amoebae from the filter stones, 5–6 stones of 1–2 cm in diameter (whole weight, 33–35 g) were washed by inverting the tube for 1 min in a 50-ml centrifuge tube with sterilized water added up to 50 ml. The washing solution was transferred to a new 50-ml centrifuge tube and the volume of the solution was adjusted to 50 ml by adding sterilized distilled water. Five millilitres of the washing solution was concentrated to 1 ml in a 15-ml centrifuge tube by centrifuging at 500 g for 5 min. The sediment was resuspended and inoculated onto a non-nutrient agar plate coated with heat-inactivated Escherichia coli. Test plates in a plastic bag were incubated at 30 °C and examined for 7 days for growth of amoebae [13].
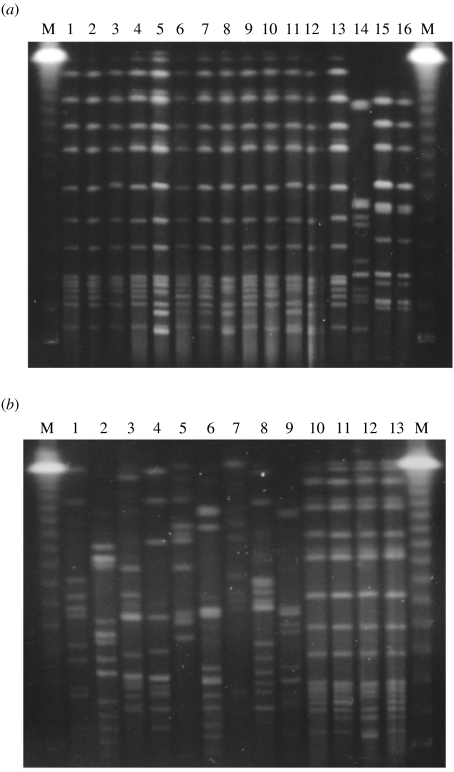
Some L. pneumophila sg 5 strains previously isolated from different origins [Fig. (b)] were used for comparison of DNA fingerprints.
Fig.
Macrorestriction analysis of chromosomal DNA derived from Legionella pneumophila sg 1 and sg 5 digested with SfiI and separated by pulsed-field gel electrophoresis. (a) A comparison of the clinical isolates with the strains isolated from water samples and swabs at different sites of the ship. Lanes 1 and 2, sg 5 isolates from patient (case 1) sputum; lane 3, sg 5 isolate from men‘s spa water; lanes 4–8, sg 5 isolates from natural stones in men’s spa filter; lanes 9–12, sg 5 isolates from natural stones in women’s spa filter; lane 13, sg 5 isolate from a strainer of women’s spa; lane 14, sg 5 isolate from a whirlpool spa; lane 15, sg 1 isolate from a swab at a strainer for women’s spa; lane 16, sg 1 isolate from natural stones in women’s spa filter. (b) L. pneumophila sg 5 strains. Lane 1, NIIB 412, Osaka LG02-11 from a spa bath; lane 2, ATCC 33216 Dallas 1E from a cooling tower; lane 3, NIIB 98 (EY 3420), a clinical isolate in Osaka [22]; lane 4, NIIB104 (EY 3427), a clinical isolate in Kurashiki; lane 5, ATCC 33737 U8W from shower head water; lane 6, NIIB 288 Ishioka 1-2-4 from a spa bath [18]; lane 7, NIIB 330 (ThaiNIH 7811) from a cooling tower; lane 8, NIIB 361 (ThaiNIH 10723) from a cooling tower; lane 9, corresponding to lane 14 of panel (a); lane 10, corresponding to lane 1 of panel (a); lane 11, corresponding to lane 4 of panel (a); lane 12, corresponding to lane 5 of panel (a); lane 13, corresponding to lane 6 of panel (a). Ms are DNA size markers, lambda ladders (Bio-Rad, Richmond, CA, USA), as indicated on the right and left sides of each electrophoregram.
RESULTS
Environmental investigations
The circulating system in the men’s indoor spa was identical to but independent of that in the women’s indoor spa. Each bathtub contained 5·5 m3 of water. The water in the tubs was changed twice a day. According to the test report of the inspection company on water samples collected on 23 December before the first cruise, water samples from the indoor men’s spa and a whirlpool spa on the deck had yielded legionellae, while water from a royal cabin bath had not (Table); but that fact was unfortunately not reported to the ship’s company until after the cruise. Because the indoor spa water showed high contamination by legionellae (1·5 ×104 c.f.u./100 ml, a spa for males), these spas were disinfected by circulation of water with a high concentration of chlorine (5–7 mg/l) for 7 h [9], resulting in decreased L. pneumophila sg 5 c.f.u. in water samples collected from an indoor (male) spa and a whirlpool spa on 14 January. However, legionellae were still not eradicated from the spas, and subsequent samples exhibited a legionella concentration of 8·0×10 to 2·9×103 c.f.u./100 ml (Table).
Table.
Isolation and characterization of clinical and environmental Legionella strains with the cruise ship
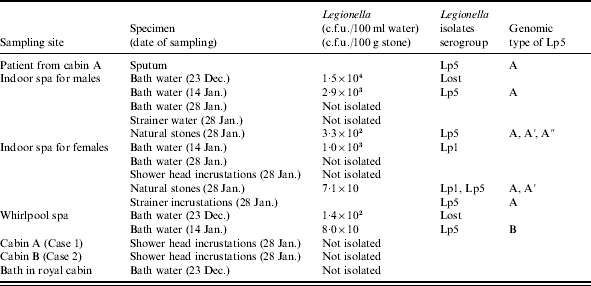
Lp1 and Lp5; L. pneumophila serogroups 1 and 5 respectively. Genomic type A, A′, A″ and B; PFGE patterns shown in lanes 1, 5, 7, and 14 in Figure (a) respectively.
After a passenger was diagnosed with Legionnaires’ disease, administrative inspection of the ship was begun and environmental samples were collected from different sites at the spas and in the male patient’s cabin on 28 January. Of the environmental water samples collected on 14 and 28 January, most were negative or soon became negative as a result of the crew’s and others’ cleaning efforts. The surfaces of the porous stones appeared clean, and no amoebae, often a reservoir of legionellae, were isolated from the stones. But some legionellae were detected in the water surrounding the stones during transit to the testing, and sonication dislodged large amounts of legionellae from inside the porous stones. As a result, it was found that some cleaning of the stones had been achieved, but not eradication of legionella. All 36 isolates from men’s spa stones were L. pneumophila sg 5, 14 of 15 isolates from women’s spa stones were L. pneumophila sg 5, with one isolate sg 1. All other isolates from the men’s spa and women’s spa were identified as L. pneumophila sg 5, and a mixture of L. pneumophila sg 1 and sg 5 respectively.
L. pneumophila subtyping
We obtained isolates from only case 1. Genomic fingerprints (PFGE) of L. pneumophila sg 5 isolates from the patient and various sites of the ship’s spas show that the same fingerprint strains as the clinical isolates (lanes 1 and 2) were obtained [Fig. (a)] from stones in both spa filters for men (lane 4) and women (lane 10) and from the men’s spa water (lane 3). Interestingly, genomic fingerprints of stone isolates revealed a small variation [lanes 4–12, Fig. (a)]. Some isolates showing one or two additive band(s) with one band’s disappearance in PFGE patterns were observed, suggesting that the porous stones harboured a group of strains derived from a common ancestor. The fingerprints of a L. pneumophila sg 5 strain from the water of the whirlpool spa and of a L. pneumophila sg 1 strain from the women’s spa water (lanes 14 and 15 respectively) were different from the fingerprint of the clinical isolates.
We examined whether the variation in PFGE patterns of L. pneumophila sg 5 strains is wide enough to discriminate between clinical and environmental isolates. As shown in Figure (b), each L. pneumophila sg 5 isolate from different origins showed a unique fingerprint, corresponding to the results of a previous report on a cluster of nosocomial Legionnaires’ disease caused by L. pneumophila sg 5 [1,4], while the clinical and the environmental isolates in this outbreak showed indistinguishable or quite similar PFGE patterns.
DISCUSSION
Legionnaires’ disease has been linked to passenger-ship cruises in several reports, but the source of the infection was clearly demonstrated only in two clusters [5, 6]. In the present cluster, we established that the source of infection was the ship’s spa baths rather than the cabin’s shower heads or the royal cabin’s bath water. Cultures of spa water samples, a strainer swab, and natural stones in filters of the spas yielded L. pneumophila sg 1 and sg 5. By analysis with PFGE, the L. pneumophila sg 5 isolates from one patient were indistinguishable from the spa water isolates. All three cases were urinary antigen positive. One male patient’s illness was confirmed to be due to L. pneumophila sg 5 infection by culture; the other male patient’s illness was presumed to be due to L. pneumophila sg 5 infection because only L. pneumophila sg 5 was detected in the men’s spa. Whether the causative agent of the female’s pneumonia belonged to sg 1 or sg 5 could not be determined because antigen of sg 5 can cross-react with that of sg 1 in the urine antigen tests. Our results of positive urines and negative cultures (cases 2 and 3) are not rare. Generally, obtaining an adequate sputum specimen from patients with Legionnaires’ disease is difficult and sensitivity is much higher in urinary antigen detection than in culture [15]. Ruf et al. reported that only two respiratory secretions were culture-positive among 13 urine-positive pneumonia patients [16]. In most situations, the use of both the urinary antigen test plus sputum culture is the best combination.
This cluster has relevant clinical implications for physicians. First, community physicians should always inquire about recent travel as part of their patient’s history and consider the possibility of legionellosis, because the disease is often overlooked as a cause of community-acquired pneumonia [5]. Second, urinary antigen detection is a rapid and easy test and can detect most cases of legionellosis caused by L. pneumophila sg 1 and others as shown in this outbreak. However, without isolation of clinical strains, the source of infection cannot be definitely confirmed [1]. Therefore, clinical specimens for Legionella isolation should always be cultured.
The results of this investigation have broad public health implications. Recently, maintenance of spa baths and adherence to sanitation standards have been advocated for prevention of Legionnaires’ disease in Japan and other countries. Such methodology is now common at hotels and bathhouses after some outbreaks of Legionnaires’ disease were attributed to spa water [17, 18], but not yet on ships. The cruise company had never performed surveillance for Legionella in the ship; the surveillance on 23 December was performed for the first time after inquiries by a regional public health centre about the probability of Legionella contamination. In the Japanese guidelines established by the Ministry of Health, Labour and Welfare, legionellae should not be detectable in bath water, with a sensitivity threshold of 10 c.f.u./100 ml. Exact requirements vary from prefecture to prefecture, but generally any detection of legionellae must be reported to a public health centre, which will require disinfection and retesting or closure.
Given the fact that the spas were implicated as the source of infection on this cruise ship, the implementation of preventive measures is imperative. Cruise-ship companies must be aware of the risk of legionellosis associated with the presence of legionellae in the ships’ spas, and must ensure that their drains and pipework are as short as possible for easier cleaning. Moreover, they must maintain all spa apparatus fastidiously. In this cluster, Legionella contamination of spa water occurred in spite of the water being changed twice a day during the cruises. One reason might be the porous natural stones in filters where biofilms harbouring legionellae and other microbes are formed. Porous natural stones or ceramic balls in filters maintained poorly are so dangerous that they should be discarded. Extracts from natural stone Maifanshi and ceramic balls in domestic spa filters [19] and a slurry of water and sand taken from a whirlpool spa sand filter [6] harboured far more legionellae than bath water. The current most common ways of disinfecting them include superheat and hyperchlorination. Another reason is the presence of about 200 l of residual water in pipework between a heater on one floor and a bathtub several floors higher without appropriate drains. Such structural defects in spa design teach us lessons. Although the chlorine concentration of the indoor spas was set to 4 mg/l with automatic dosing devices, the actual concentration of free available chlorine residuals in the spa water had not been measured and was presumed to have been much lower than that in the guidelines of the Ministry of Health, Labour and Welfare (0·2–0·4 mg/l, <1·0 mg/l).
The concentration of Legionella spp. in water specimens obtained from the ship’s indoor spa water ranged from 80 to 15000 c.f.u./100 ml, a concentration comparable to those of previous large outbreaks in spas [18, 20] (8·4×103 c.f.u./100 ml and 8·0×103 c.f.u./100 ml). Legionnaires’ disease is an opportunistic infection and frequently affects people who are elderly, smoke cigarettes, or have other underlying disease [21]. Our three cases were all 70 years or older, and the index case was a heavy smoker with mild emphysema. In this cluster, however, there were few confirmed cases of Legionnaires’ disease although many elderly people participated in these cruises. One probable explanation is that the indoor spas have no air compressors and therefore fewer aerosols. Another explanation may be that physicians did not order laboratory testing for the diagnosis of suspected cases.
In conclusion, this report is the first documented cluster of L. pneumophila sg 5 infection associated with a cruise ship and is the first cluster of Legionnaires’ disease associated with a cruise ship in Japan. Since legionellosis cases associated with cruise ships have probably been overlooked, and advanced age is a risk factor for the disease [21], it is necessary to pay close attention to the hygiene of water supply systems, air-conditioning apparatus, and especially spas in any cruise ship where elderly people are likely to be passengers. We strongly recommend that porous, incompletely washable stones should not be used in a spa system.
ACKNOWLEDGEMENTS
We thank Eiko Yabuuchi, Department of Microbial Bioinformatics, Gifu University School of Medicine, Wantana Paveenkittiporn, NIH, Nonthaburi, Thailand, and Kyoko Masuko, Ibaraki Prefectural Institute of Public Health for L. pneumophila sg 5 strains; Hiroshi Hagiwara, Health, Welfare and Environmental Services Department, Hyogo Prefecture, for providing information on the problems and the improvement of the spa facility on the ship.
DECLARATION OF INTEREST
None.
REFERENCES
- 1.Benin AL, Benson RF, Besser RE. Trends in legionnaires disease, 1980–1998: declining mortality and new patterns of diagnosis. Clin Infect Dis. 2002;35:1039–1046. doi: 10.1086/342903. [DOI] [PubMed] [Google Scholar]
- 2.National Institute of Infectious Diseases and Tuberculosis and Infectious Diseases Control Division, Ministry of Health, Labour and Welfare. Legionellosis, April 1999–December 2002, Japan. http://idsc.nih.go.jp/iasr/24/276/tpc276.html. IASR Infectious Agents Surveillance Report. 2003;24:27–28. ). Accessed 3 Aug. 2005. [Google Scholar]
- 3.Rowbotham TJ. Legionellosis associated with ships: 1977 to 1997. Commun Dis Public Health. 1998;1:146–151. [PubMed] [Google Scholar]
- 4.Jalaludin BB, Nguyen OTK, Goldthorpe IC, Chiew RF. Legionnaires’ disease: co-infection with Legionella pneumophila serogroups 1 and 5. Med J Aust. 1997;166:277–278. doi: 10.5694/j.1326-5377.1997.tb140117.x. [DOI] [PubMed] [Google Scholar]
- 5.Castellani Pastoris M, Lo Monaco R, Goldoni P et al. Legionnaires’ disease on a cruise ship linked to the water supply system: clinical and public health implications. Clin Infect Dis. 1999;28:33–38. doi: 10.1086/515083. [DOI] [PubMed] [Google Scholar]
- 6.Jernigan DB, Hofmann J, Cetron MS et al. Outbreak of Legionnaires’ disease among cruise ship passengers exposed to a contaminated whirlpool spa. Lancet. 1996;347:494–499. doi: 10.1016/s0140-6736(96)91137-x. [DOI] [PubMed] [Google Scholar]
- 7.Kobayashi A, Yamamoto Y, Chou S, Hashimoto S. Severe Legionella pneumophila pneumonia associated with the public bath on a cruise ship in Japan. J Anesth. 2004;18:129–131. doi: 10.1007/s00540-003-0218-0. [DOI] [PubMed] [Google Scholar]
- 8.Sugishita Y. Legionnaires’ disease on a cruise ship to and from Taiwan. Case 2 – Tokyo [in Japanese]. IASR Infectious Agents Surveillance Report. 2004;25:40–41. [Google Scholar]
- 9.Takemoto M, Gonda H, Oishi T, Yamamoto K, Ikenouchi N. A case of legionellosis on a domestic cruise ship [in Japanese] IASR Infectious Agents Surveillance Report. 2004;25:41–42. [Google Scholar]
- 10.Li GS, Wang JX, Jing L. The anti-fluoride effects of Zhonghua Maifanshi. An experimental study. Fluoride. 1990;23:37–42. [Google Scholar]
- 11.Okuda K, Ikedo M, Yabuuchi E. Wadowsky-Yee-Okuda (WYO) medium: a new selective medium for the isolation of legionellae strains from environmental water specimens [in Japanese] Kansenshogaku Zasshi. 1984;58:1073–1082. doi: 10.11150/kansenshogakuzasshi1970.58.1073. [DOI] [PubMed] [Google Scholar]
- 12.Amemura-Maekawa J, Kura F, Watanabe H, Gondaira F, Sugiyama J., Marre R, Abu Kwaik Y, Bartlett C. Legionella. Washington, DC: ASM Press; 2002. Analysis of Legionella pneumophila serogroup 1 isolates in Japan by using pulsed-field gel electrophoresis and monoclonal antibodies pp. 302–304. [Google Scholar]
- 13.Kuroki T, Sata S, Yamai S, Yagita K, Katsube Y, Endo T. Occurrence of free-living amoebae and Legionella in whirlpool baths [in Japanese] Kansenshogaku Zasshi. 1998;72:1056–1063. doi: 10.11150/kansenshogakuzasshi1970.72.1056. [DOI] [PubMed] [Google Scholar]
- 14.Chang FY, Jacobs SL, Colodny SM, Stout JE, Yu VL. Nosocomial Legionnaires’ disease caused by Legionella pneumophila serogroup 5: laboratory and epidemiologic implications. J Infect Dis. 1996;174:1116–1119. doi: 10.1093/infdis/174.5.1116. [DOI] [PubMed] [Google Scholar]
- 15.Murdoch DR. Diagnosis of Legionella infection. Clin Infect Dis. 2003;36:64–69. doi: 10.1086/345529. [DOI] [PubMed] [Google Scholar]
- 16.Ruf B, Schurmann D, Horbach I, Fehrenbach FJ, Pohle HD. Prevalence and diagnosis of Legionella pneumonia: a 3-year prospective study with emphasis on application of urinary antigen detection. J Infect Dis. 1990;162:1341–1348. doi: 10.1093/infdis/162.6.1341. [DOI] [PubMed] [Google Scholar]
- 17.Sugiyama K, Nishio T, Goda Y et al. An outbreak of legionellosis linked to bath water circulating through a filter at a spa resort, March–April 2000 – Shizuoka. http://idsc.nih.go.jp/iasr/21/247/de2471.html. IASR Infectious Agents Surveillance Report. 2000;21:188. ). Accessed 3 Aug. 2005. [Google Scholar]
- 18.Nakamura H, Yagyu H, Kishi K et al. A large outbreak of legionnaires’ disease due to an inadequate circulating and filtration system for bath water–epidemiologic manifestations. Intern Med. 2003;42:806–811. doi: 10.2169/internalmedicine.42.806. [DOI] [PubMed] [Google Scholar]
- 19.Takahashi T, Yabuuchi E, Endo T, Furuhata K. Sanitary problems of 24 h-bath and administrative countermeasures for them [in Japanese] Jap J Environ Infect. 1998;13:129–136. [Google Scholar]
- 20.Fallon RJ, Rowbotham TJ. Microbiological investigations into an outbreak of Pontiac fever due to Legionella micdadei associated with use of a whirlpool. J Clin Pathol. 1990;43:479–483. doi: 10.1136/jcp.43.6.479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Marston BJ, Lipman HB, Breiman RF. Surveillance for legionnaires’ disease. Risk factors for morbidity and mortality. Arch Intern Med. 1994;154:2417–2422. [PubMed] [Google Scholar]
- 22.Fujita I, Tsuboi H, Ohotsuka M et al. Legionella dumoffii and Legionella pneumophila serogroup 5 isolated from 2 cases of fulminant pneumonia [in Japanese] Kansenshogaku Zasshi. 1989;63:801–810. doi: 10.11150/kansenshogakuzasshi1970.63.801. [DOI] [PubMed] [Google Scholar]