Abstract
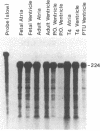
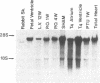
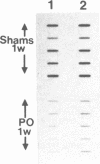
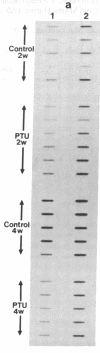
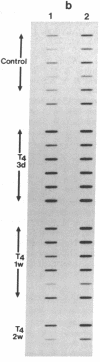
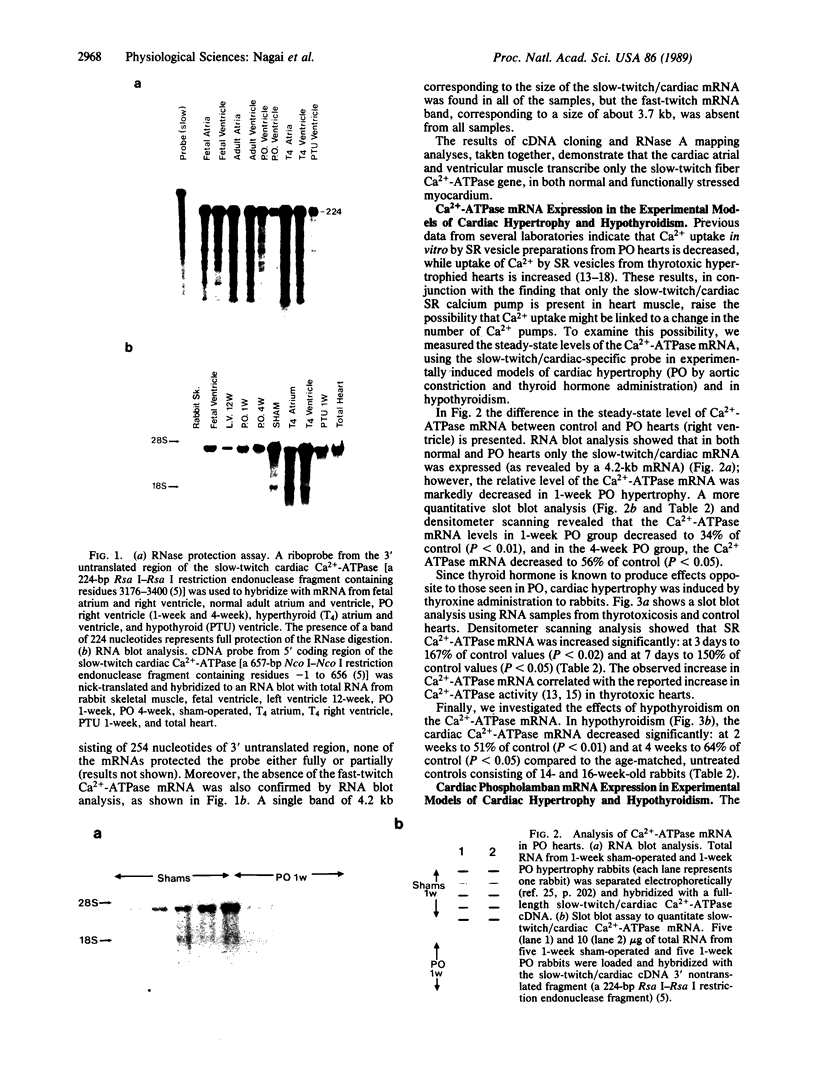
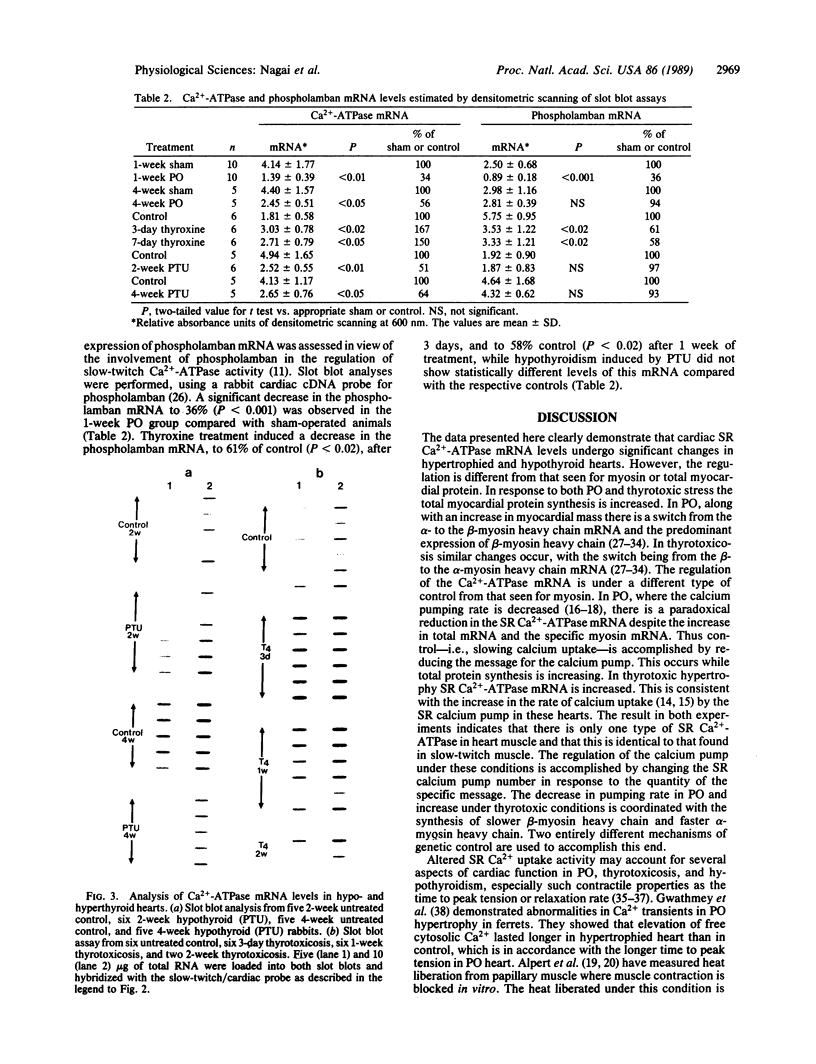
The sarcoplasmic reticulum (SR) and the contractile protein myosin play an important role in myocardial performance. Both of these systems exhibit plasticity--i.e., quantitative and/or qualitative reorganization during development and in response to stress. Recent studies indicate that SR Ca2+ uptake function is altered in adaptive cardiac hypertrophy and failure. The molecular basis (genetic and phenotypic) for these changes is not understood. In an effort to determine the underlying causes of these changes, we characterized the rabbit cardiac Ca2+-ATPase phenotype by molecular cloning and ribonuclease A mapping analysis. Our results show that the heart muscle expresses only the slow-twitch SR Ca2+-ATPase isoform. Second, we quantitated the steady-state mRNA levels of two major SR Ca2+ regulatory proteins, the Ca2+-ATPase and phospholamban, to see whether changes in mRNA content might provide insight into the basis for functional modification in the SR of hypertrophied hearts. In response to pressure overload hypertrophy, the relative level of the slow-twitch/cardiac SR Ca2+-ATPase mRNA was decreased to 34% of control at 1 week. The relative Ca2+-ATPase mRNA level increased to 167% of control after 3 days of treatment with thyroid hormone. In contrast, in hypothyroid animals, the relative Ca2+-ATPase mRNA level decreased to 51% of control at 2 weeks. The relative level of phospholamban mRNA was decreased to 36% in 1-week pressure overload. Hyperthyroidism induced a decrease to 61% in the phospholamban mRNA level after 3 days of treatment, while hypothyroidism had virtually no effect on phospholamban mRNA levels. These data indicate that the expression of SR Ca2+-ATPase and phospholamban mRNA may not be coordinately regulated during myocardial adaptation to different physiological conditions.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blanchard E. M., Mulieri L. A., Alpert N. R. The effects of acute and chronic inotropic interventions on tension independent heat of rabbit papillary muscle. Basic Res Cardiol. 1987;82 (Suppl 2):127–135. doi: 10.1007/978-3-662-11289-2_13. [DOI] [PubMed] [Google Scholar]
- Brandl C. J., Green N. M., Korczak B., MacLennan D. H. Two Ca2+ ATPase genes: homologies and mechanistic implications of deduced amino acid sequences. Cell. 1986 Feb 28;44(4):597–607. doi: 10.1016/0092-8674(86)90269-2. [DOI] [PubMed] [Google Scholar]
- Brandl C. J., deLeon S., Martin D. R., MacLennan D. H. Adult forms of the Ca2+ATPase of sarcoplasmic reticulum. Expression in developing skeletal muscle. J Biol Chem. 1987 Mar 15;262(8):3768–3774. [PubMed] [Google Scholar]
- Eichhorn P., Grimm J., Koch R., Hess O., Carroll J., Krayenbuehl H. P. Left ventricular relaxation in patients with left ventricular hypertrophy secondary to aortic valve disease. Circulation. 1982 Jun;65(7):1395–1404. doi: 10.1161/01.cir.65.7.1395. [DOI] [PubMed] [Google Scholar]
- Everett A. W., Sinha A. M., Umeda P. K., Jakovcic S., Rabinowitz M., Zak R. Regulation of myosin synthesis by thyroid hormone: relative change in the alpha- and beta-myosin heavy chain mRNA levels in rabbit heart. Biochemistry. 1984 Apr 10;23(8):1596–1599. doi: 10.1021/bi00303a002. [DOI] [PubMed] [Google Scholar]
- Fujii J., Lytton J., Tada M., MacLennan D. H. Rabbit cardiac and slow-twitch muscle express the same phospholamban gene. FEBS Lett. 1988 Jan 18;227(1):51–55. doi: 10.1016/0014-5793(88)81412-1. [DOI] [PubMed] [Google Scholar]
- Fujii J., Ueno A., Kitano K., Tanaka S., Kadoma M., Tada M. Complete complementary DNA-derived amino acid sequence of canine cardiac phospholamban. J Clin Invest. 1987 Jan;79(1):301–304. doi: 10.1172/JCI112799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grossman W., McLaurin L. P. Diastolic properties of the left ventricle. Ann Intern Med. 1976 Mar;84(3):316–326. doi: 10.7326/0003-4819-84-3-316. [DOI] [PubMed] [Google Scholar]
- Gunteski-Hamblin A. M., Greeb J., Shull G. E. A novel Ca2+ pump expressed in brain, kidney, and stomach is encoded by an alternative transcript of the slow-twitch muscle sarcoplasmic reticulum Ca-ATPase gene. Identification of cDNAs encoding Ca2+ and other cation-transporting ATPases using an oligonucleotide probe derived from the ATP-binding site. J Biol Chem. 1988 Oct 15;263(29):15032–15040. [PubMed] [Google Scholar]
- Gwathmey J. K., Morgan J. P. Altered calcium handling in experimental pressure-overload hypertrophy in the ferret. Circ Res. 1985 Dec;57(6):836–843. doi: 10.1161/01.res.57.6.836. [DOI] [PubMed] [Google Scholar]
- Hamrell B. B., Alpert N. R. The mechanical characteristics of hypertrophied rabbit cardiac muscle in the absence of congestive heart failure: the contractile and series elastic elements. Circ Res. 1977 Jan;40(1):20–25. doi: 10.1161/01.res.40.1.20. [DOI] [PubMed] [Google Scholar]
- Harigaya S., Schwartz A. Rate of calcium binding and uptake in normal animal and failing human cardiac muscle. Membrane vesicles (relaxing system) and mitochondria. Circ Res. 1969 Dec;25(6):781–794. doi: 10.1161/01.res.25.6.781. [DOI] [PubMed] [Google Scholar]
- Hoh J. F., McGrath P. A., Hale P. T. Electrophoretic analysis of multiple forms of rat cardiac myosin: effects of hypophysectomy and thyroxine replacement. J Mol Cell Cardiol. 1978 Nov;10(11):1053–1076. doi: 10.1016/0022-2828(78)90401-7. [DOI] [PubMed] [Google Scholar]
- Inesi G. Mechanism of calcium transport. Annu Rev Physiol. 1985;47:573–601. doi: 10.1146/annurev.ph.47.030185.003041. [DOI] [PubMed] [Google Scholar]
- Izumo S., Lompré A. M., Matsuoka R., Koren G., Schwartz K., Nadal-Ginard B., Mahdavi V. Myosin heavy chain messenger RNA and protein isoform transitions during cardiac hypertrophy. Interaction between hemodynamic and thyroid hormone-induced signals. J Clin Invest. 1987 Mar;79(3):970–977. doi: 10.1172/JCI112908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korczak B., Zarain-Herzberg A., Brandl C. J., Ingles C. J., Green N. M., MacLennan D. H. Structure of the rabbit fast-twitch skeletal muscle Ca2+-ATPase gene. J Biol Chem. 1988 Apr 5;263(10):4813–4819. [PubMed] [Google Scholar]
- Lamers J. M., Stinis J. T. Defective calcium pump in the sarcoplasmic reticulum of the hypertrophied rabbit heart. Life Sci. 1979 Jun 18;24(25):2313–2319. doi: 10.1016/0024-3205(79)90529-0. [DOI] [PubMed] [Google Scholar]
- Limas C. J. Calcium transport ATPase of cardiac sarcoplasmic reticulum in experimental hyperthyroidism. Am J Physiol. 1978 Dec;235(6):H745–H752. doi: 10.1152/ajpheart.1978.235.6.H745. [DOI] [PubMed] [Google Scholar]
- Litten R. Z., 3rd, Martin B. J., Low R. B., Alpert N. R. Altered myosin isozyme patterns from pressure-overloaded and thyrotoxic hypertrophied rabbit hearts. Circ Res. 1982 Jun;50(6):856–864. doi: 10.1161/01.res.50.6.856. [DOI] [PubMed] [Google Scholar]
- Lompre A. M., Schwartz K., d'Albis A., Lacombe G., Van Thiem N., Swynghedauw B. Myosin isoenzyme redistribution in chronic heart overload. Nature. 1979 Nov 1;282(5734):105–107. doi: 10.1038/282105a0. [DOI] [PubMed] [Google Scholar]
- Lompré A. M., Nadal-Ginard B., Mahdavi V. Expression of the cardiac ventricular alpha- and beta-myosin heavy chain genes is developmentally and hormonally regulated. J Biol Chem. 1984 May 25;259(10):6437–6446. [PubMed] [Google Scholar]
- Lytton J., MacLennan D. H. Molecular cloning of cDNAs from human kidney coding for two alternatively spliced products of the cardiac Ca2+-ATPase gene. J Biol Chem. 1988 Oct 15;263(29):15024–15031. [PubMed] [Google Scholar]
- MacLennan D. H., Brandl C. J., Korczak B., Green N. M. Amino-acid sequence of a Ca2+ + Mg2+-dependent ATPase from rabbit muscle sarcoplasmic reticulum, deduced from its complementary DNA sequence. Nature. 1985 Aug 22;316(6030):696–700. doi: 10.1038/316696a0. [DOI] [PubMed] [Google Scholar]
- Nagai R., Pritzl N., Low R. B., Stirewalt W. S., Zak R., Alpert N. R., Litten R. Z. Myosin isozyme synthesis and mRNA levels in pressure-overloaded rabbit hearts. Circ Res. 1987 May;60(5):692–699. doi: 10.1161/01.res.60.5.692. [DOI] [PubMed] [Google Scholar]
- Rohrer D., Dillmann W. H. Thyroid hormone markedly increases the mRNA coding for sarcoplasmic reticulum Ca2+-ATPase in the rat heart. J Biol Chem. 1988 May 25;263(15):6941–6944. [PubMed] [Google Scholar]
- Sordahl L. A., McCollum W. B., Wood W. G., Schwartz A. Mitochondria and sarcoplasmic reticulum function in cardiac hypertrophy and failure. Am J Physiol. 1973 Mar;224(3):497–502. doi: 10.1152/ajplegacy.1973.224.3.497. [DOI] [PubMed] [Google Scholar]
- Suko J. The calcium pump of cardiac sarcoplasmic reticulum. Functional alterations at different levels of thyroid state in rabbits. J Physiol. 1973 Feb;228(3):563–582. doi: 10.1113/jphysiol.1973.sp010100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tada M., Katz A. M. Phosphorylation of the sarcoplasmic reticulum and sarcolemma. Annu Rev Physiol. 1982;44:401–423. doi: 10.1146/annurev.ph.44.030182.002153. [DOI] [PubMed] [Google Scholar]
- Taylor R. R., Covell J. W., Ross J., Jr Influence of the thyroid state on left ventricular tension-velocity relations in the intact, sedated dog. J Clin Invest. 1969 Apr;48(4):775–784. doi: 10.1172/JCI106035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitmer J. T., Kumar P., Solaro R. J. Calcium transport properties of cardiac sarcoplasmic reticulum from cardiomyopathic Syrian hamsters (BIO 53.58 and 14.6): evidence for a quantitative defect in dilated myopathic hearts not evident in hypertrophic hearts. Circ Res. 1988 Jan;62(1):81–85. doi: 10.1161/01.res.62.1.81. [DOI] [PubMed] [Google Scholar]