SUMMARY
From August 2002 to February 2003 25 cases of hepatitis A were notified from one town in Jutland. The first cases were children of three families who returned from an endemic country. The infection spread subsequently in the local community and within households. A case-control study among household index cases showed that hepatitis A was associated with contact to a case in an after-school group (OR 29·6) and with contact to a case household member in a school class or day-care centre group (OR 9·5). From a serosurvey it was estimated that for each notified patient approximately one additional infection has occurred in the households. The infection was imported by children of immigrants, born in Denmark, returning from a visit to friends and relatives in the high-incidence country of origin of their parents and was then propagated through contact between children in after-school groups, schools and their families. Immunoprophylaxis should be given to children prior to visits to friends and relatives in endemic countries and to case contacts.
INTRODUCTION
In Denmark, acute hepatitis A (HAV) is a mandatory notifiable disease. The incidence declined in recent years from 5·5/100 000 inhabitants in 1980 (n=282) to 1·2/100 000 in 2001 (n=63). Most (68%) of the patients in 2001 were <20 years old and the incidence was highest in children <10 years. The incidence in immigrants (11·4/100 000) was higher than in persons of Danish origin (0·4/100 000). In 70% of the cases, the infection was acquired abroad [1].
Starting in week 31 of 2002, the Medical Office of Health (MOH) of Ringk⊘bing County, Jutland, and the Department of Epidemiology at the Statens Serum Institut (SSI) noticed an increase in HAV cases reported from Holstebro. Holstebro, the second largest town in the predominantly rural Ringk⊘bing County in Northern Denmark, has a population of 41 094, of which ∼5% are immigrants. Between 1996 and 2001, a total of three cases of HAV, aged between 41 and 60 years, had been notified from Holstebro.
The notified patients were mostly children and their parents. The MOH of Ringk⊘bing County organized a campaign with public health nurses who visited schools and child-care centres in Holstebro in autumn 2002 to emphasize the need for strict hygiene measures such as supervised hand washing. They also explained the transmission pattern of HAV to parents and institution staff members. Nevertheless, the outbreak continued.
This paper describes the epidemiology and investigation of this outbreak and makes a case for a critical review of the current guidelines regarding prophylactic interventions against HAV infection in Denmark.
METHODS
Descriptive study
In Denmark, passive surveillance of HAV is done through notification of patients by general practitioners and hospitals to the local MOH and the Department of Epidemiology of SSI. Therefore, general practitioners in Holstebro were asked to submit any missing notifications as well as to notify any new cases immediately. In addition, we conducted active case finding by searching the records from the laboratory responsible for HAV diagnosis for Ringk⊘bing County for additional anti-HAV IgM-positive patients during the period of interest. From the notification forms submitted by the patients’ general practitioner, we collected the age, gender and place of birth of all notified cases and date of onset as well as any reported exposure to other patients or attendance of the patient or another household member to a school or child-care centre.
Analytical study
We conducted a retrospective case-control study to assess risk factors for HAV infection among household index cases during this outbreak. A household index case was defined as a resident of Holstebro who had experienced clinical symptoms of HAV infection between August 2002 and February 2003 together with a positive anti-HAV IgM test result. When multiple patients had been reported from one household, we selected the individual who had the earliest onset of disease in his/her household. For each household index case we randomly selected four controls, who were matched to cases by date of birth, from the Danish civil registration system among the residents of Holstebro. Cases and controls were interviewed by telephone using a standard questionnaire. Data collected included HAV vaccination status, human normal immunoglobulin (HNIG) administration, contact with a case or a case family member in a school class or day-care centre group or after-school group, country of origin, travel to a country with high HAV incidence, consumption of water from private wells and contact with diapered children. Adult study participants were furthermore asked to report any injecting drug use and sexual contacts between men (MSM). The period under study was the 2 months preceding the onset of HAV of the index case. The data were analysed using Epi-Info 2002 (CDC, Atlanta, GA, USA) and SAS software (SAS Institute Inc., Cary, NC, USA). The Mantel–Haenszel odds ratio (ORMH) and the attributable fraction (AF) were calculated for each risk factor. A conditional multivariable logistic regression analysis was carried out. As there was no substantial difference between estimates obtained from matched and unmatched analysis the matching was broken for the logistic regression in order to obtain better convergence in the model.
Serological survey
We carried out a serological survey in April 2003 in order to measure the prevalence of anti-HAV antibodies in recently affected and unaffected classes and day-care groups. Recently affected classes/groups were defined as classes/groups attended by a notified HAV case with a first positive anti-HAV IgM test result within 3 months prior to the survey date or attended by a household member of such a case. Oral fluid samples were collected from all children present in these classes on the sampling day. For each affected class, an unaffected parallel class as well as one corresponding class from a school in Holstebro, which had not been involved in the outbreak, were sampled. In addition, we collected an oral fluid sample from all members of the households from which a patient had been notified during this outbreak. Attendants of the selected classes and day-care groups, who had been notified during this outbreak, were sampled in their respective households. The oral fluid samples were analysed by enzyme-immunosorbant assay (EIA) at the Health Protection Agency, Colindale, UK [2]. We calculated the seroprevalence (% anti-HAV-positive samples) in affected and unaffected classes on the basis of anti-HAV IgM or IgG presence.
Environmental investigations
We reviewed the control samples of the municipality water supply routinely submitted to the public health services for indicators of any contamination during the outbreak period.
RESULTS
Descriptive study
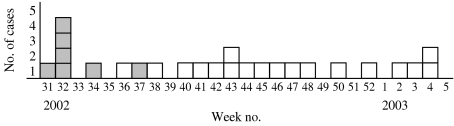
Between week 31 of 2002 and week 7 of 2003 25 HAV patients were notified from Holstebro, representing an incidence increase from 1·2/100 000 (mean of previous 6 years) to 51·2/100 000 inhabitants in 2002 (Fig. 1). The patients came from 16 different households. There were five households with  2 patients, accounting for 14 notified patients, and 11 households with only one notified patient. The patients involved in this outbreak were from 4 to 15 (61%) and 29–48 years old (39%). Fourteen of the HAV patients were male, 11 were female (Fig. 2). Among the first patients with onset of symptoms between 2 August 2002 and 11 February 2002 were seven children, aged between 4 and 15 years, of three families who had travelled together to a country with a high incidence of HAV from 20 June to 1 August 2002, visiting and relatives. The parents of these children had immigrated to Denmark from that country many years ago whereas the children were born and raised in Denmark. From the notification forms it appeared that the patients either clustered in families or shared schools or child care with other known patients or their family members. Six of the HAV patients were admitted to hospital, among them four children aged 4, 5, 10 and 14 years.
2 patients, accounting for 14 notified patients, and 11 households with only one notified patient. The patients involved in this outbreak were from 4 to 15 (61%) and 29–48 years old (39%). Fourteen of the HAV patients were male, 11 were female (Fig. 2). Among the first patients with onset of symptoms between 2 August 2002 and 11 February 2002 were seven children, aged between 4 and 15 years, of three families who had travelled together to a country with a high incidence of HAV from 20 June to 1 August 2002, visiting and relatives. The parents of these children had immigrated to Denmark from that country many years ago whereas the children were born and raised in Denmark. From the notification forms it appeared that the patients either clustered in families or shared schools or child care with other known patients or their family members. Six of the HAV patients were admitted to hospital, among them four children aged 4, 5, 10 and 14 years.
Fig. 1.
Notified cases of hepatitis A by week of onset, Holstebro, Denmark, August 2002 to February 2003 ( , travelled to country with high hepatitis A incidence from 20 June to 1 August 2003).
, travelled to country with high hepatitis A incidence from 20 June to 1 August 2003).
Fig. 2.
Age and gender distribution of hepatitis A cases notified from Holstebro, Denmark, August 2002 to February 2003.
Analytical study
Of the 16 household index cases and the 64 controls selected to participate in this study, all agreed to be interviewed. Two cases and one control were interviewed during a visit to their home due to logistic or language reasons; all others were interviewed by telephone. The mean age difference between cases and controls was 0·9 days (range 0–5 days), the female–male ratio among cases was 1·29 and 0·88 among controls. Ten of the index cases were children (62·5%) and six (37·5%) were adults. The age of the cases ranged from 4 to 48 years. Parents answered on behalf of study participants <5 years of age.
None of the study participants had been vaccinated against HAV nor received HNIG prior to the outbreak. Similarly, no injecting drug use or MSM contacts were reported. Cases were more likely than controls to attend the same school class/day-care group as a case household member (ORMH 13·75, 95% CI 1·5–125·2, AF 0·9), having parents born in a country with high HAV incidence (ORMH 6·0, 95% CI 1·4–25·5, AF 0·8), and attending the same after-school group as a case (ORMH∝) (Table 1).
Table 1.
Cases of hepatitis A and controls by contact and travel history, Holstebro, Denmark, August 2002 to February 2003
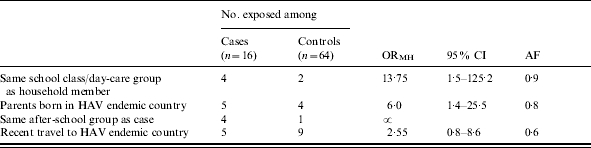
ORMH, Mantel–Haenszel odds ratio; CI, confidence interval, AF, attributable fraction.
In the multivariable logistic regression model cases were more likely than controls to attend the same after-school group as a case (OR 29·6, 95% CI 2·8–310·2) and to attend the same school class/day-care group as a case household member (OR 9·5, 95% CI 1·3–69·1). In an analysis restricted to children, cases were more likely than controls to attend the same after-school group as a case (OR 55·4, 95% CI 3·9–785·6) and to have parents born in a country with high HAV incidence (OR 24·4, 95% CI 3·1–190·1) (Table 2).
Table 2.
Adjusted odds for contact and origin among hepatitis A cases and controls, Holstebro, Denmark, August 2002 to February 2003

OR, Odds ratio; CI, confidence interval.
Serological survey
Of the attendants of classes and day-care groups identified for the serological survey, with the exception of a pupil who was unable to participate due to ill health, all present on the sampling day participated. A total of 476 oral fluid samples, representing a response rate of 99·8% were collected from infants and toddlers in four day-care institutions and children in four schools. Of these samples 174 were collected from nine groups and classes attended by a case or a case family member and 302 samples were collected from 16 groups and classes not attended by a case or a case family member. The seroprevalence in exposed classes and groups was 2·87% compared to 2·32% in unexposed classes. In day-care groups attended by a case family member the seroprevalence was 5·26% and in day-care groups not attended by a case family member it was 1·92% (Table 3). However, when adjusted for parent origin, the difference disappeared (data not shown). No results indicating acute or late acute stages of HAV infection were found among the school and day-care group attendants sampled.
Table 3.
Prevalence of anti-HAV IgG in classes attended by hepatitis A cases or case family members and control classes, Holstebro, Denmark, August 2002 to February 2003
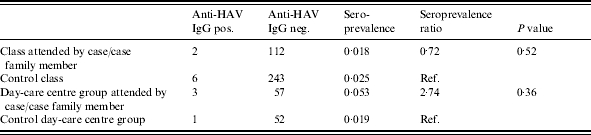
IgG, Gamma Globulin.
A total of 75 members of households affected during this outbreak were sampled. Five of the 24 notified persons sampled (21%) were anti-HAV IgM-positive, indicating an acute/late acute stage of HAV infection. Of the 51 household members that had not been notified, 26 (51%) were anti-HAV IgG positive. Eight of these were adult immigrants from HAV endemic countries and most likely had been infected prior to this outbreak. Based on this assumption, we estimate that the serosurvey identified 18 additional infected individuals in these households. In other words, for each notified patient almost one additional infection had occurred in the households (Table 4).
Table 4.
Stages of HAV infection among hepatitis A case household members by age group, notification status and origin, Holstebro, Denmark, August 2002 to February 2003

Environmental investigations
Holstebro is supplied with unchlorinated water from wells that are between 70 m and 90 m deep. During the period from August 2002 to February 2003 no water samples submitted under the regular water hygiene-monitoring scheme showed any unacceptable level of contaminants.
Discussion
The outbreak described in this paper is one of the largest known outbreaks of HAV in Denmark since surveillance for HAV was initiated in 1980. The findings of this investigation indicate that the initial infection was imported by children of immigrants visiting friends and relatives in their parents’ country of origin in which HAV is highly endemic. This applies to many HAV outbreaks observed in recent years in Denmark and other industrialized countries, where most children and young adults are now susceptible for HAV. For example, in 2001, two institutional and two family outbreaks involving seven and five cases respectively were identified in Denmark. Three of these four outbreaks could be linked to an index case infected abroad [1]. The Danish National Board of Health therefore recommends that children of immigrants be vaccinated before visiting friends and relatives in their parents’ high-incidence country of origin [3]. It is, at present, not known to what extent these recommendations are followed. None of the visitors to friends and relatives involved in this outbreak had been vaccinated prior to travelling. Currently, the costs of this vaccination have to be borne by the vaccinees. Making this vaccination cost-free under the national health insurance scheme might increase compliance and thus decrease the risk of HAV infection for all Danish residents. Apart from financial reasons, a low request for HAV vaccination could also be due to a lack of public and professional awareness of the potential seriousness of the disease.
In the outbreak reported here, following the return of these families to Denmark and the start of the new school year, the infection spread in the local community with subsequent infections in other families. Interestingly, contact in school classes seems to have contributed little to the perpetuation of this outbreak, whereas contact in the after-school groups seems to pose a special risk. After-school groups differ from school classes in as far as they are attended by school children from different classes and age groups (6–13 years), who play together with limited adult supervision until their parents return from work. In addition, after-school groups have less toilet facilities and are more crowded. These findings should be taken into consideration when identifying target groups for preventive measures during future outbreaks involving school children.
This outbreak was detected late. It could have been detected earlier if real-time reporting had been done, which can be achieved by timely reporting by clinicians combined with laboratory-based surveillance of HAV. That would have enabled an early enforcement of preventive action including immunoprophylaxis, which might have curbed the outbreak earlier.
Limitations of our study include that interviews for the case-control study were carried out regarding exposures that had taken place 2–6 months earlier. Recall bias, especially among the controls, may have led to misclassification of exposure in some instances. Moreover, the sensitivity of the anti-HAV EIA for IgM in oral fluid used in the serological survey is limited to 3 months after infection. Consequently, only classes and groups attended by the last four cases of this outbreak and their family members had oral fluid specimens collected. This may have given rise to an underestimation of the overall seroconversion of case contacts in institutions linked to this outbreak as transmission rates may have been higher in its earlier phases.
In Denmark, the current recommendations of the Danish National Board of Health for post-exposure prophylaxis in HAV outbreaks are to use HNIG. However, in recent years the question of which post-exposure prophylaxis is most effective in curtailing community outbreaks of HAV with person-to-person transmission has been under debate. HNIG is believed to modify or attenuate the clinical symptoms of HAV but not to prevent HAV infection itself [4]. It is effective in preventing cases of HAV if given soon after exposure [5] – but, in spite of its administration, some individuals have subclinical infections and may still contribute to transmission of HAV [6]. In one study, widespread use of HNIG among school and household contacts of cases led to a transient decrease in the rate of notification of new cases, but prevention of further cases was not achieved [7].
Inactivated HAV vaccine has become available since the second half of the 1990s. In a randomized trial in Italy, the protective efficacy of the HAV vaccine was 82%. All vaccinees were IgM-positive within 15 days after immunization and no serious adverse events were observed. In the unvaccinated group (n=207) 12 secondary cases arose (5·8%) while in the vaccinated group (n=197) two secondary cases were noted (1·0%). The two secondary cases in the vaccinated group showed no clinical symptoms [8]. Several authors report promising results using the vaccine as post-exposure prophylaxis in community outbreaks. In a systematic review Demicheli & Tiberti found that the effectiveness of inactivated HAV vaccine for both primary and secondary cases ranged from 82% to 95% [9]. No evidence was found in an exploratory review of evidence published in English since 1945 convincingly demonstrating that post-exposure administration of currently available HNIG is effective in preventing HAV infection and disease. However, larger trials on the effectiveness of HAV vaccine as post-exposure prophylaxis are needed, particularly to identify the critical interval between exposure and vaccine administration and the effect of vaccination on virus shedding [10].
Considering these findings it seems advisable to reconsider the current advice of the Danish National Board of Health to exclusively use HNIG for post-exposure prophylaxis in HAV outbreaks. In this context, the experiences of 17 of the 22 countries participating in Eurohep.Net, who are offering active immunization to household contacts of HAV cases for several years already, could be useful [11].
An issue of the national epidemiological bulletin, Epi-Nyt, regarding the current HAV situation in Denmark and this outbreak in particular, specifically stressing the need for immunoprophylaxis for children of immigrants before visiting friends and relatives in high-risk countries and contact immunoprophylaxis in an outbreak involving institutions, was published in August 2003 [12]. A letter has been sent by the Department of the Medical Officer of Health of Ringk⊘bing County to Holstebro municipality explaining the findings of the outbreak investigation and outlining recommendations regarding the handling of future outbreaks of HAV. In order to inform the public, a newspaper article describing the problem of children visiting friends and relatives in countries with a high incidence of infectious diseases has been published by a newspaper with a large immigrant readership in Denmark, pointing out the possibilities of obtaining suitable protection through the Danish health system prior to such visits.
Acknowledgements
The authors thank John Parry and Tamara McDonald at the Health Protection Agency, Colindale, UK, for conducting the enzyme-immunosorbant assay (EIA) on the oral fluid samples. Thanks are also due to Ellis Hansen from Ringk⊘bing Medical Office of Health for her assistance with interviewing study participants. The cooperation of Holstebro municipality in this outbreak investigation is gratefully acknowledged.
DECLARATION OF INTEREST
None.
References
- 1.Nielsen MS. 2002. p. 40. Hepatitis A 2001. EPI-NEWS.
- 2.Parry JV, Perry KR, Panday S, Mortimer PP. Diagnosis of hepatitis A and B by testing saliva. J Med Virol. 1989;28:255–260. doi: 10.1002/jmv.1890280410. [DOI] [PubMed] [Google Scholar]
- 3.Anon. 2002. Guidelines for the prevention of viral hepatitis. Danish National Board of Health. [in Danish].
- 4.Krugman S, Ward R, Giles JP, Jacobs AM. Infectious hepatitis. Studies on the effect of gamma globulin and on the incidence of apparent infection. J Am Med Assoc. 1960;174:823–830. doi: 10.1001/jama.1960.03030070001001. [DOI] [PubMed] [Google Scholar]
- 5.Stapelton JT. Host immune response to hepatitis A virus. J Infect Dis. 1995;171:9–14. doi: 10.1093/infdis/171.supplement_1.s9. [DOI] [PubMed] [Google Scholar]
- 6.Hadler SC, Erben JJ, Matthews D, Starko K, Francis DP, Maynard JE. Effect of immunoglobulin on hepatitis A in day-care centers. J Am Med Assoc. 1983;249:48–53. [PubMed] [Google Scholar]
- 7.Aszkenasy OM. A community outbreak of hepatitis A in a religious community in Indiana: failure of immune serum globulin to prevent spread of infection. Epidemiol Infect. 2000;124:309–313. doi: 10.1017/s0950268899003544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sagliocca L, Amoroso P, Stroffolini T et al. Efficacy of hepatitis A vaccine in prevention of secondary hepatitis A infection: a randomised trial. Lancet. 1999;353:1136–1139. doi: 10.1016/S0140-6736(98)08139-2. [DOI] [PubMed] [Google Scholar]
- 9.Demicheli V, Tiberti D. The effectiveness and safety of hepatitis A vaccine: a systematic review. Vaccine. 2003;21:2242–2245. doi: 10.1016/s0264-410x(03)00135-x. [DOI] [PubMed] [Google Scholar]
- 10.Taliani G, Gaeta GB. Hepatitis A: post-exposure prophylaxis. Vaccine. 2003;21:2234–2237. doi: 10.1016/s0264-410x(03)00138-5. [DOI] [PubMed] [Google Scholar]
- 11.EUROHEP.NET. 2004. http://www.eurohep.net/default.asp?p=75&l=06. http://www.eurohep.net/default.asp?p=75&l=06 – Surveillance and prevention of vaccine preventable hepatitis. Data on surveillance and prevention of hepatitis A and B in 22 countries, 1990s–2001. EUROHEP.NET. ). Accessed 10 January 2005.
- 12.Gervelmeyer A, Nielsen MS, M⊘lbak K, Frey L, Sckerl H, Damberg E. Hepatitis A 2002. EPI-NEWS. 2003:36. doi: 10.1017/S0950268805005200. [DOI] [PMC free article] [PubMed] [Google Scholar]