SUMMARY
The clinical features and microbiological characteristics of 315 patients with definite or possible infective endocarditis (IE) from January 1995 to December 2003 were evaluated. There were 187 males and 128 females with a mean age of 51 years (range, 1 month to 92 years). Ninety-three patients (30%) had a diagnosis of valvular heart disease and 24 (8%) had received prosthetic valve replacement. Blood culture was negative in 62 patients (20%). Staphylococci (91 patients, 32%), including methicillin-susceptible Staphylococcus aureus (15%), methicillin-resistant S. aureus (11%), and coagulase-negative staphylococci (6%), were the most commonly encountered pathogens followed by viridans group streptococci (77 patients, 24%). Eight patients (25%) had various neurological, renal, embolic, and cardiac complications. Patients with neurological complications [odds ratio (OR) 8·175, P<0·001], nosocomial IE (OR 6·661, P<0·001), underlying malignancy (OR 4·993, P<0·001), elevated serum creatinine level (OR 3·132, P=0·001), or elevated WBC count (>15 000/mm3) (OR 2·537, P=0·007) were at significantly increased risk of mortality. This study found mortality from IE was associated with several factors, among which neurological complications were the most hazardous. Patients with more than one risk factor had poorer prognosis. These results suggest the need for more aggressive management in patients with IE when multiple risk factors for mortality are identified.
INTRODUCTION
Infective endocarditis (IE) was always fatal in the pre-antibiotic era [1]. Despite advances in antibiotic therapy and valve surgery in recent decades, and the widely adoption of transoesophageal echocardiography (TEE) as the primary diagnostic modality, the reported in-hospital mortality rate from IE remains high (14–31%) [2–7]. The majority (55–75%) of patients with native valve endocarditis have predisposing conditions including rheumatic heart disease, congenital heart disease, mitral valve prolapse, degenerative heart disease, asymmetrical septal hypertrophy, or intravenous drug abuse [8, 9]. In patients with IE, development of conditions including heart failure, neurological complications, renal failure, fungal infection, valve ring or myocardial abscesses or with prosthetic valve IE has frequently been associated with poor prognosis [10–12].
Numerous studies had been conducted to elucidate the factors contributing to the high mortality and morbidity rates of patients with IE [7, 13–17]. Wallace et al. [7] found that among clinical, microbiological, and echocardiographic features, only white blood cell (WBC) count and serum albumin were independent predictors of short-term death. Cabell et al. [13] reported an association between infection with Staphylococcus aureus and death. Hasbun et al. [14] found that comorbid illness, mental status, heart failure, causative organism, and surgical therapy were important factors determining 6-month survival. Mourvillier et al. [15] reported that septic shock, cerebral emboli, immunocompromised state, and cardiac surgery independently predict the outcome of native valve IE, while the outcome of prosthetic valve IE was associated with septic shock, neurological complications, and immunocompromised states. Prognosis is good in young intravenous drug abusers with S. aureus infection of the tricuspid valve [16, 17]. There has been a lack of large-scale study of IE in Taiwan and few retrospective studies of its prognostic determinants [18, 19].
The goal of this study was to delineate the clinical profile of IE in patients from Taiwan, and to identify the risk factors for mortality by multivariate analysis. The results of this study may have implications for treatment strategy which could improve prognosis.
PATIENTS and METHODS
Study population
We reviewed the medical records of all the patients with a discharge diagnosis of IE at National Taiwan University Hospital (Taipei, Taiwan), a 2000-bed tertiary care hospital, from January 1995 to December 2003. Data collected for each patient included age, sex, chief complaint, presumptive diagnosis on admission, underlying cardiac or other medical illness (congenital heart disease, valvular heart disease, diabetes, hypertension, old stroke, chronic kidney disease, chronic liver disease, chronic lung disease, haematological malignancies, other malignant diseases, and autoimmune disease), history of IE, prosthetic valve, and intravenous drug abuse. WBC count, C-reactive protein (CRP), serum albumin, renal function test, and liver function test results obtained on admission or just before the manifestations of nosocomial IE noted during hospitalization were also recorded. In addition, echocardiographic findings (vegetation site and size), causative organisms identified by blood culture, and complications associated with IE, including neurological, renal, cardiac, and embolic complications were also evaluated.
Definitions
All included patients fulfilled the modified Duke criteria for definite or possible IE, as proposed by Li et al. [20]. Renal dysfunction was defined as a serum creatinine level >1·5 mg/dl (133 μmol/l), and liver dysfunction as elevation of liver enzymes by more than twice the upper limit of normal [aspartate aminotransferase (AST) >74 U/l (1·23 μkat/l), alanine aminotransferase (ALT) >82 U/l (1363 μkat/l)]. Neurological complications included cerebral emboli or ischaemic stroke, mycotic aneurysm with or without leading to cerebral haemorrhage, and cerebral abscess. Renal complications included acute renal failure, defined as an increase of serum creatinine by >0·5 mg/dl (44 μmol/l) during admission, or glomerulonephritis. Embolic complications included splenic infarct or abscess, coronary embolism with myocardial infarction, pulmonary embolism, and peripheral limb embolization, but cerebral embolization was not included. Cardiac complications included new atroventricular conduction block, intracardiac abscess, and heart failure.
Statistical analysis
All analyses were performed using the Statistical Package for the Social Science (SPSS for Windows 11.0, SPSS Institute, Chicago, IL, USA). In univariate analyses, Pearson’s χ2 test and Fisher’s exact test were used for comparison of categorical variables between fatal and non-fatal cases, while the Student’s t test was used for analysis of continuous variables. Homogeneity of variance was assessed by Levene’s test. All variables that were identified as significant indicators of mortality in the univariate analysis were included in the multivariate analysis with stepwise linear regression. All tests were two-tailed, and a P value of <0·05 was considered to be statistically significant.
RESULTS
Characteristics of the study population
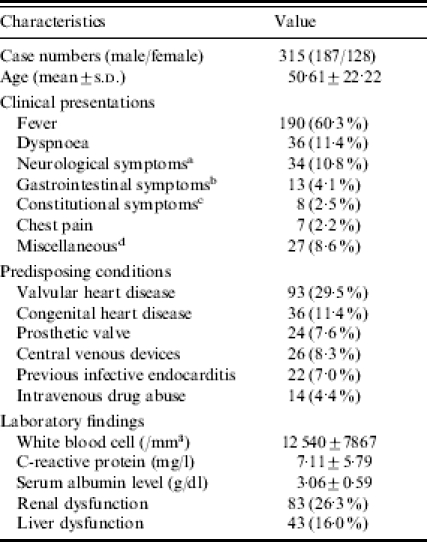
Within the 9-year period of study, there were 346 patients who had a discharge diagnosis of IE. Among these patients, 31 were excluded because their clinical conditions did not fulfil the study criteria of definite or possible IE. The remaining 315 patients were included in the analysis. Table 1 shows the demographic characteristics, underlying diseases, and clinical features of the patients. There were 187 males and 128 females; male-to-female ratio 1·46. The mean age was 50·6 years, ranging from 1 month to 92 years.
Table 1.
Demographics, underlying diseases, and clinical features of 315 patients with infective endocarditis at National Taiwan University Hospital from 1995 to 2003
Neurological symptoms included limb weakness, headache, seizure, or change in consciousness.
Gastrointestinal symptoms included diarrhea, nausea, vomiting, or abdominal pain.
Constitutional symptoms included fatigue, general malaise, or loss of body weight.
Miscellaneous symptoms included other cardiovascular symptoms (arrhythmia or conduction disturbance, or palpitation, other than chest pain and dyspnoea), musculoskeletal symptoms (arthralgia, muscle ache, or non-specific limb pain), pedal oedema, haematological symptoms (petechiae, bleeding).
Diabetes mellitus was present in 47 patients (14·9%), and hypertension in 59 (18·7%). Valvular heart disease was found in 93 patients (29·5%) and was the most commonly encountered predisposing factor in adults. Twenty-four patients (7·6%) had received valve replacement with a prosthetic valve. Fourteen patients (4·4%) were intravenous drug abusers. Central venous devices, including port-A catheter, pacemaker or double-lumen catheter were placed before the development of IE in 26 patients (8·25%).
On admission, patients had an average WBC count of 12 540±7867 (1/mm3), mean CRP of 7·11±5·79 (mg/l), and mean serum albumin of 3·06±0·59 g/dl (30·6±5·9 g/l). Renal dysfunction was noted in 83 patients (26·34%). Forty-three patients (15·99%) had liver dysfunction. Fever was the most commonly encountered chief complaint. The frequency of other major manifestation at presentation was detailed in Table 1.
Thirty-six patients (11·4%) had congenital heart disease, which was the most frequent predisposing factor in children. The most frequent types of congenital heart diseases included: ventricular septal defects in 13 patients (36·1%), including two patients with concomitant ventricular septal defect and patent ductus arteriosus, complex congenital heart disease in seven patients (19·4%), tetralogy of Fallot in five patients (13. 9%), and bicuspid aortic valve in four patients (11·1%). Other identified congenital anomalies included patent ductus arteriosus (8·3%), atrial septal defect (5·6%), Marfan syndrome (2·8%), and arteriovenous fistula (2·8%).
Echocardiographic findings included vegetations noted on the mitral valve in 129 patients (41·0%), on the aortic valve in 116 patients (36·8%), on the tricuspid valve in 41 patients (13·0%), on the pulmonic valve in 10 patients (3·2%), and had vegetations noted on other sites in 12 patients (3·8%), including left or right ventricular outflow tract, interventricular septum, on the wall of pulmonary artery, or pacemaker lead. Less than one-tenth of patients had negative findings on echocardiography (28 patients, 8·9%). The mean vegetation size was 13·47 ±6·63 mm. IE was classified as definite in 212 patients and as possible in 103 patients. The mortality rate was 21·6%. All the nine patients with Streptococcus bovis bacteraemia underwent colon fibreoscopy examinations. Among these patients, one had rectal cancer, six had colorectal adenoma, and one each had rectal xanthoma and internal haemorrhoid.
Table 2 shows the microbiological spectrum of patients with IE. Streptococci and staphylococci were found with equal frequency, with each noted in 97 patients (30·8%). Viridans streptococci were the most common causative Streptococcus spp. responsible for IE in 77 patients (24·4%). Methicillin-susceptible S. aureus (MSSA) was the most common causative Staphylococcus spp. with methicillin-resistant S. aureus (MRSA) more prevalent than coagulase-negative staphylococci at rates of 35·1% and 5·7% of isolates respectively. Seventeen patients (5·4%) were infected with enterococci, and another 17 patients were infected with Gram-negative bacilli. There were five patients (1·59%) infected by HACEK group bacteria. Sixty-two patients (19·7%) had negative blood cultures.
Table 2.
Causative microorganisms yielded on blood cultures from 315 patients with infective endocarditis at National Taiwan University Hospital from 1995 to 2003

HACEK, Haemophilus parainfluenzae, Haemophilus aphrophilus, Cardiobacterium hominis, Eikenella corrodens, and Kingella spp.
Complications developed in 80 patients (25·4%). These included neurological complications in 31 patients, the most common of which was embolic stroke (21 patients). Subarachnoid haemorrhage due to rupture of mycotic aneurysm was also documented in 7 patients. Twenty-three (7·3%) patients had embolic events, which presented as splenic infarct in 8 patients, distal limb emboli in 8 patients, or pulmonary embolism in 7 patients. Cerebral embolism was classified as a neurological complication. Seventeen patients (5·4%) had renal complications, and acute renal failure and glomerulonephritis were the most common causes. Cardiac complications were noted in 17 patients (5·4%). Myocardial infarction developed in 2 patients due to coronary arterial embolization. IE involving the aortic valve was associated with a higher rate of cardiac complication (9·2% vs. 3·1%, P=0·037). This association was at least partially due to the anatomical contiguity of the aortic valve and conduction system. Tricuspid valve involvement was associated with a lower mortality rate (9·8%), possibly due to a reduced rate of neurological complications (0% vs. 16·9%, P=0·021). The deposition of vegetation emboli in the pulmonary circulation might explain the reduced rate of neurological complications unless paradoxical embolism due to intracardiac right-to-left shunt occurred.
Characteristics of culture-positive vs. culture-negative patients
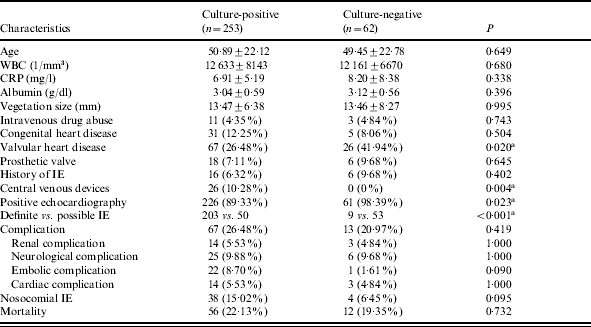
Blood culture results were positive in 253 patients (80·3%) and negative in 62 patients (19·7%). Comparison of these two groups by univariate analysis revealed that culture-negative patients had a significantly higher rate of diagnosis of valvular heart disease (26·5% vs. 41·9%, P=0·020). This might have been because positive blood culture is one of the requirements, either as a major or a minor criterion, for defining IE with the modified Duke criteria. Culture-positive patients had a higher rate of placement of central venous devices (10·3% vs. 0%, P=0·004). Most culture-positive patients met the criteria for definite IE, while most culture-negative patients only met the criteria for possible IE. This is easily explained by the fact that positive blood culture was one of the criteria used to define IE. Other parameters, such as clinical characteristics, underlying medical illness, site and size of vegetations, or laboratory findings such as WBC count, CRP, and serum albumin on admission were not associated with differences in the of yield rate of blood culture. There was also no significant difference in the complication rate or mortality rate between these two groups of patients (Table 3).
Table 3.
Characteristics of 315 patients with infective endocarditis according to the results of blood culture
IE, Infective endocarditis.
P<0·05.
Predictors for mortality
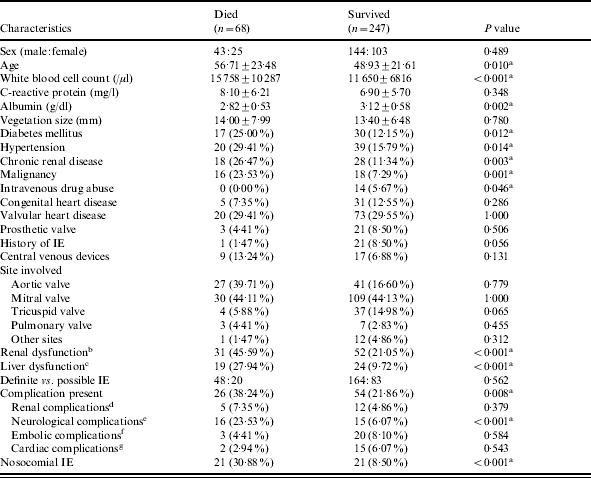
Univariate analysis was used to compare the characteristics of the 68 patients (21·6%) who died and 247 patients who survived (Table 4). There were many factors that showed a significant correlation with mortality including: advanced age (56·7 vs. 48·9 years, P=0·010), diabetes mellitus (25·0% vs. 12·2%, P=0·012), hypertension (29·4% vs. 15·79%, P=0·014), chronic renal disease (26·5% vs. 11·3%, P=0·003), and malignancy (23·5% vs. 7·3%, P=0·001). Laboratory examination on admission showed that mortality rate was significantly associated with higher WBC count (15 758 vs. 12 540/mm3), lower serum albumin level (2·82 vs. 3·12 g/l, P=0·002), and renal or liver dysfunction (45·6% vs. 21·05% and 27·9% vs. 9·7% respectively, both P<0·001). Neurological complications and nosocomial IE were also associated with a higher mortality rate (both P<0·001). The only predictor of lower mortality rate was intravenous drug abuse, but this association was of marginal significance (0% vs. 5·7%, P=0·046).
Table 4.
Univariate analysis of outcome for 315 patients with infective endocarditis
IE, Infective endocarditis.
P<0·05.
Indicates serum creatinine level higher than 1·5 mg/dl on admission or at the occurrence of nosocomial IE.
Indicates elevated liver enzyme of more than two times normal (AST>74 U/l, ALT>82 U/l).
Indicate acute renal failure defined by increase of serum creatinine level by more than 0·5 mg/dl during admission, and glomerulonephritis.
Indicate cerebral emboli or ischaemic stroke, mycotic aneurysm with or without leading to cerebral haemorrhage, or cerebral abscess.
Indicate splenic infarct or abscess, coronary embolism with myocardial infarction, pulmonary embolism, and peripheral limb embolization. Cerebral embolization was not included.
Indicate conduction disturbance, intracardiac abscess, and heart failure.
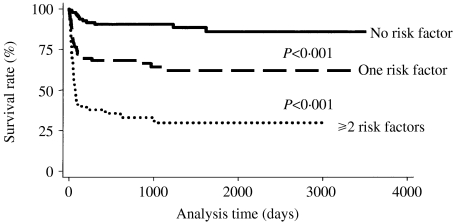
The independent risk factors for mortality identified in the multivariate analysis were neurological complications (OR 8·175, P<0·001), nosocomial IE (OR 6·661, P<0·001), underlying malignancy (OR 4·993, P<0·001), renal dysfunction (OR 3·132, P=0·001), and elevated WBC count (OR 2·537, P=0·007) (Table 5). Low serum albumin level (<3 g/l) (OR 1·643, P=0·138) and advanced age (>65 years) (OR 1·472, P=0·255) were not significantly related to mortality. As shown in the Figure, the Kaplan–Meier survival curves for patients without risk factors were significantly different from those with one risk factor (P<0·001), and this difference was also significant between those with one and those with two or more risk factors. The differences in survival were mainly due to risk factors which developed in the early stage of disease.
Table 5.
Multivariate logistic regression for risk factors of mortality in 315 patients with infective endocarditis at National Taiwan University Hospital from 1995 to 2003
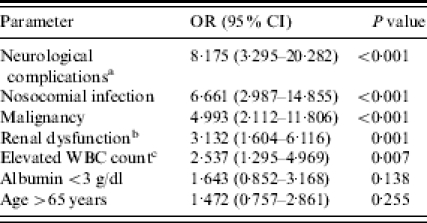
OR, Odds ratio; CI, confidence interval.
Indicates limb weakness, headache, seizure, or change in consciousness.
Indicates serum creatinine level higher than 1·5 mg/dl on admission or at the occurrence of nosocomial IE.
Indicates WBC count >15 000/μl.
Fig.
Kaplan–Meier survival curves for patients with infective endocarditis according to number of risk factors.
DISCUSSION
IE remains the fourth most important life-threatening infectious syndrome [21]. The overall mortality rates for both native-valve and prosthetic-valve endocarditis are as high as 20–25%, with death resulting primarily from central nervous system embolic events and haemodynamic deterioration [22]. There have been few multivariate analyses of the prognostic determinants in IE [23–27], and only one report including Chinese patients (80 cases) has been published in medical literature [26].
Mortality correlates in the univariate analysis included age, WBC count, serum albumin level, diabetes, hypertension, chronic renal disease, malignancy, previous history of IE, renal or liver dysfunction, neurological complication and nosocomial IE. Intravenous drug addiction was associated with a significantly reduced mortality rate, compatible with a previous report [28], and might be related to younger age, less concomitant comorbidity, and a higher rate of tricuspid involvement. Tricuspid IE was associated with a trend towards lower mortality rate, but this difference was not significant. Its impact on mortality was masked by its positive effect on neurological complications. These factors were included in multivariate logistic regression analysis, which identified variables independently associated with nosocomial IE, presence of neurological complications, malignancy, renal dysfunction, and high WBC count. Nosocomial IE was associated with worse clinical condition, more comorbidity, neurological complications, and subsequent sequelae. Thus, our study found that nosocomial IE and neurological complications were the greatest contributors to mortality in patients with IE. A previous study found that neurological complications were independent markers for mortality in patients with IE [29].
Studies, which evaluated the ability of echocardiography to predict complications of IE, revealed that vegetation size was a predictor of embolic events [27, 30]. Jaffe et al. [25] reported a trend towards a higher risk of embolization in patients with vegetations >10 mm. However this echocardiographic finding did not predict worse outcome or death in our study. Embolization to sites other than the brain might have a more minor negative effect on prognosis. Fewer patients with prosthetic IE included in our study and the Framingham criteria adopted by Chu et al. for detecting early heart failure or heart failure unrelated to IE might be the cause for a lower rate of cardiac complication than that previously reported [31].
Underlying malignancy also predicted worse outcome due to poorer baseline condition or haematological and immunological damage following chemotherapy. In this study age only showed a trend towards a higher mortality rate, while a previous study in southern Taiwan found age to be an independent predictor on multivariate analysis [19]. These conflicting data may reflect selection bias or differences in the definition of elderly patients [32–35].
Serum albumin is an acute phase reactant indicating inflammation, as well as WBC count. Factors responsible for changes of albumin level in inflammation include haemodilution, increased vascular permeability, increased local consumption, and decreased synthesis due to inhibition by cytokines. Changes in these parameters with more prominent inflammation may explain their association with worse outcome. However, only high WBC count was a significant risk factor for mortality in this study. Impaired renal function implicates underlying renal insufficiency, worse haemodynamics, or immunological or embolic complications caused by IE, and thus, may also result in worse outcome.
There were several limitations to our study. First, a lower proportion of streptococci causing IE and more culture-negative cases than most recent endocarditis cohorts are of interest. Information about antibiotic exposure prior to IE diagnosis is important and would be helpful to explain these phenomena. However, a large proportion of the patients in this study were transferred from other local hospitals or outpatient clinics. However, such information was not available for the majority of our patients. Many patients might have received antibiotic treatment (prescribed by the physicians in local hospitals or outpatient clinics) before they were admitted to our hospital. Second, our data was from patients treated at a tertiary referral centre, which suggests a selection bias towards patients with greater illness severity, and higher rates of complications and mortality. Third, because this study was conducted at a single centre, regional and institutional variation in the microbiology, diagnosis and treatment of IE may have influenced results. Finally, this was an observational study, and data on all clinical parameters were obtained by retrospective review of medical records. In addition, some data were unavailable. These situations may have led to detection bias. Further studies about other factors influencing outcomes of patients with IE, such as different regimens of antibiotic therapy, types of causative organisms, and timing of administration of appropriate antibiotics and surgical intervention (valve replacement), are needed.
In conclusion, consideration of risk factors for mortality in patients with IE is important to determine the appropriate treatment. Obtaining at least three sets of blood culture for patients with suspected IE before any antibiotics are started is crucial. An aggressive treatment strategy and earlier intervention before the development of complications appears to be indicated in high-risk patients.
DECLARATION OF INTEREST
None.
REFERENCES
- 1.Kerr AJ. Subacute bacterial endocarditis. Springfield, IL: Charles C Thomas; 1955. [Google Scholar]
- 2.Richardson JV, Karp RB, Kirklin JW, Dismukes WE. Treatment of infective endocarditis: a 10-year comparative analysis. Circulation. 1978;58:589–597. doi: 10.1161/01.cir.58.4.589. [DOI] [PubMed] [Google Scholar]
- 3.D’Agostino RS, Miller DC, Stinson EB et al. Valve replacement in patients with native valve endocarditis: what really determines operative outcome. Ann Thorac Surg. 1985;40:429–438. doi: 10.1016/s0003-4975(10)60097-5. [DOI] [PubMed] [Google Scholar]
- 4.Arbulu A, Asfaw I. Management of infective endocarditis: seventeen years experience. Ann Thorac Surg. 1987;43:144–149. doi: 10.1016/s0003-4975(10)60383-9. [DOI] [PubMed] [Google Scholar]
- 5.Mullany CJ, McIsaacs AI, Rowe MH et al. The surgical treatment of infective endocarditis. World J Surg. 1989;13:132–136. doi: 10.1007/BF01671175. [DOI] [PubMed] [Google Scholar]
- 6.Hoen B, Alla F, Selton-Suty C et al. Changing profile of infective endocarditis: results of a 1-year survey in France. J Am Med Assoc. 2002;288:75–81. doi: 10.1001/jama.288.1.75. [DOI] [PubMed] [Google Scholar]
- 7.Wallace SM, Walton BI, Kharbanda RK et al. Mortality from infective endocarditis: clinical predictors of outcome. Heart. 2002;88:53–60. doi: 10.1136/heart.88.1.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Arvay A, Lengyel M. Incidence and risk factors of prosthetic valve endocarditis. Eur J Cardiothorac Surg. 1988;2:340–346. [PubMed] [Google Scholar]
- 9.Calderwood SB, Swinski LA, Waternaux CM et al. Risk factors for the development of prosthetic valve endocarditis. Circulation. 1985;72:31–37. doi: 10.1161/01.cir.72.1.31. [DOI] [PubMed] [Google Scholar]
- 10.Cates JE, Christie RV. Subacute bacterial endocarditis: a review of 442 patients treated in 14 centres appointed by the Penicillin Trials Committee of Medical Research Council. Q J Med. 1951;20:93–130. [PubMed] [Google Scholar]
- 11.Ahern H. Cellular responses to oxidative stress: extensively studied bacterial systems provide insights into more complex systems and, potentially, human diseases. ASM News. 1991;57:627–630. [Google Scholar]
- 12.Lu VL, Fang GD, Keys TF et al. Prosthetic valve endocarditis: superiority of surgical valve replacement versus medical therapy only. Ann Thorac Surg. 1994;58:1073–1077. doi: 10.1016/0003-4975(94)90458-8. [DOI] [PubMed] [Google Scholar]
- 13.Cabell CH, Pond KK, Peterson GE et al. The risk of stroke and death in patients with aortic and mitral valve endocarditis. Am Heart J. 2001;142:75–80. doi: 10.1067/mhj.2001.115790. [DOI] [PubMed] [Google Scholar]
- 14.Hasbun R, Vikram HR, Barakat LA et al. Complicated left-sided native valve endocarditis in adults: risk classification for mortality. J Am Med Assoc. 2003;289:1933–1940. doi: 10.1001/jama.289.15.1933. [DOI] [PubMed] [Google Scholar]
- 15.Mourvillier B, Trouillet JL, Timsit JF et al. Infective endocarditis in the intensive care unit: clinical spectrum and prognostic factor in 228 consecutive patients. Intensive Care Med. 2004;30:2046–2052. doi: 10.1007/s00134-004-2436-9. [DOI] [PubMed] [Google Scholar]
- 16.Chambers HF, Korzeniowski OM, Sande MA National Collaborative Endocarditis Study group. Staphylococcus aureus endocarditis: clinical manifestations in addicts and nonaddicts. Medicine. 1983;62:170–177. [PubMed] [Google Scholar]
- 17.Korzeniowski O, Sande MA. Combination antimicrobial therapy for Staphylococcus aureus endocarditis in patients addicted to parenteral drugs and in nonaddicts: a prospective study. Ann Intern Med. 1982;97:496–503. doi: 10.7326/0003-4819-97-4-496. [DOI] [PubMed] [Google Scholar]
- 18.Weng MC, Chang FY, Young TG et al. Analysis of 109 cases of infective endocarditis in a tertiary care hospital. Chinese Med J. 1996;58:18–23. [PubMed] [Google Scholar]
- 19.Chao TH, Li YH, Tsai WC et al. Prognostic determinants of infective endocarditis in the 1990s. J Formos Med Assoc. 1999;98:474–479. [PubMed] [Google Scholar]
- 20.Li JS, Sexon DJ, Mick N et al. Proposed modifications to the Duke Criteria for the diagnosis of infective endocarditis. Clin Infect Dis. 2000;30:633–638. doi: 10.1086/313753. [DOI] [PubMed] [Google Scholar]
- 21.Bayer AS, Bolger AF, Taubert KA et al. AHA scientific statement: diagnosis and management of infective endocarditis and its complications. Circulation. 1998;98:2936–2948. doi: 10.1161/01.cir.98.25.2936. [DOI] [PubMed] [Google Scholar]
- 22.Mylonakis E, Calderwood SB. Infective endocarditis in adults. N Engl J Med. 2001;345:1318–1330. doi: 10.1056/NEJMra010082. [DOI] [PubMed] [Google Scholar]
- 23.Mansur AJ, Ginberg M, Cardoso RHA et al. Determinants of prognosis in 300 episodes of infective endocarditis. Thorac Cardiovasc Surg. 1996;44:2–10. doi: 10.1055/s-2007-1011974. [DOI] [PubMed] [Google Scholar]
- 24.Schulz R, Werner GS, Fuchs JB et al. Clinical outcome and echocardiographic findings of native and prosthetic valve endocarditis in the 1990s. Eur Heart J. 1996;17:281–288. doi: 10.1093/oxfordjournals.eurheartj.a014846. [DOI] [PubMed] [Google Scholar]
- 25.Jaffe WM, Morgan DE, Pearlman AS et al. Infective endocarditis, 1983–1988: echocardiographic findings and factors influencing morbidity and mortality. J Am Coll Cardiol. 1990;15:1227–1233. doi: 10.1016/s0735-1097(10)80005-1. [DOI] [PubMed] [Google Scholar]
- 26.Woo KS, Lam YM, Kwok HT et al. Prognostic index in prediction of mortality from infective endocarditis. Int J Cardiol. 1989;24:47–54. doi: 10.1016/0167-5273(89)90040-5. [DOI] [PubMed] [Google Scholar]
- 27.Sanfilippo AJ, Picard MH, Newwell JB et al. Echocardiographic assessment of patients with infective endocarditis: prediction of risk for complications. J Am Coll Cardiol. 1991;18:1191–1199. doi: 10.1016/0735-1097(91)90535-h. [DOI] [PubMed] [Google Scholar]
- 28.Mathew J, Addai T, Anand A, Morrobel A, Maheshwari P, Freels S. Clinical features, site of involvement, bacteriologic findings, and outcome of infective endocarditis in intravenous drug users. Arch Intern Med. 1995;155:1641–1648. [PubMed] [Google Scholar]
- 29.Di Salvo G, Thuny F, Rosenberg V et al. Endocarditis in the elderly: clinical, echocardiographic, and prognostic features. Eur Heart J. 2003;24:1576–1583. doi: 10.1016/s0195-668x(03)00309-9. [DOI] [PubMed] [Google Scholar]
- 30.Di Salvo G, Habib G, Pergola V et al. Echocardiography predicts embolic events in infective endocarditis. J Am Coll Cardiol. 2001;37:1069–1076. doi: 10.1016/s0735-1097(00)01206-7. [DOI] [PubMed] [Google Scholar]
- 31.Chu VH, Cabell CH, Benjamin DK et al. Early predictors of in-hospital death in infective endocarditis. Circulation. 2004;109:1745–1749. doi: 10.1161/01.CIR.0000124719.61827.7F. [DOI] [PubMed] [Google Scholar]
- 32.Selton-Suty C, Hoen B, Grentzinger A et al. Clinical and bacteriological characteristics of infective endocarditis in the elderly. Heart. 1997;77:260–263. doi: 10.1136/hrt.77.3.260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Werner GS, Schulz R, Fuchs JB et al. Infective endocarditis in the elderly in the era of transesophageal echocardiography: clinical feature and prognosis compared with younger patients. Am J Med. 1996;100:90–97. doi: 10.1016/s0002-9343(96)90017-0. [DOI] [PubMed] [Google Scholar]
- 34.Gagliardi JP, Nettles RE, McCarty DE et al. Native valve infective endocarditis in the elderly and younger patients: comparison of clinical features and outcomes with use of Duke criteria and the duke endocarditis database. Clin Infect Dis. 1998;26:1165–1168. doi: 10.1086/520304. [DOI] [PubMed] [Google Scholar]
- 35.Netzer ROM, Zollinger E, Seiler C et al. Native valve infective endocarditis in elderly and younger patients: comparison of clinical feature and outcome with the use of Duke criteria. Clin Infect Dis. 1998;26:933–934. doi: 10.1086/517229. [DOI] [PubMed] [Google Scholar]