SUMMARY
Hepatitis C virus (HCV) and hepatitis B virus (HBV) are highly prevalent, often co-occuring infections among drug users. We examined HBV prevalence and risk behaviour patterns among a group of HCV-negative heroin and/or cocaine users in order to understand HBV risk and prevention opportunities among this unique group. Of 164 people enrolled, 44% had injected drugs. Overall, 24% of participants tested positive for exposure to HBV; drug injectors (28%) were only slightly and not significantly (P=0·287) more likely to test positive than those who had never injected drugs (21%). HBV exposure was significantly associated with multiple indicators of greater sex risk. HBV status was not associated with any demographic characteristic, but participants who reported longer duration of cocaine use were significantly less likely to test positive to exposure for HBV. It appears that HBV risk among HCV-negative drug users in this cohort is primarily due to sexual behaviour.
INTRODUCTION
The incidence of hepatitis B virus (HBV) has declined steadily in the United States since the early 1990s [1], when the Centers for Disease Control and Prevention (CDC) recommended routine childhood immunization as part of their Healthy People 2000 objectives [2, 3]. However, with the focus of federal resources on childhood immunization, those adults most at risk for HBV – drug users, persons with multiple sexual partners, men who have sex with men, and incarcerated persons – continue to account for more than two-thirds of all new HBV infections in the United States [4].
The high prevalence of markers of HBV infection (HBcAb or sAg) among injection drug users (IDUs) is well documented, ranging from 30 to 80% [5–10]. The CDC estimates that 40% of IDUs become infected with HBV within the first year of drug use, and over 80% are infected within 10 years [5]. However, only 34% of the nation’s estimated 5 million heroin users report injecting [11], and users of crack and powdered cocaine are more likely to be non-injection drug users (NIDUs). Despite its relevance to many of the nation’s drug users, limited research has focused on the prevalence of past and present HBV infection among NIDUs. Available data seems to indicate that NIDUs are at increased risk when compared to the general population, primarily as a result of high-risk sexual behaviour [12–14].
As part of a serosurvey among young adults in a high-risk neighbourhood, researchers in New York found that prevalence of past HBV infection increased with ‘hardest drug ever used’, implying that while IDUs were at highest risk, NIDUs such as people who smoked or snorted were also at substantial risk [12]. Kuo and colleagues studied a cohort of more than 300 IDUs and NIDUs in Baltimore, and found the overall prevalence of past HBV infection to be 30%. IDUs were more likely to have been infected than NIDUs (37% vs. 19%), but prevalence among NIDUs was still substantially higher than among the general population. NIDUs who were female, who spent more than 8 h each day on the street, and who had been sexually active for more than 11 years were more likely than those who did not have those characteristics to have evidence of past infection. The overall prevalence of past HBV vaccination was only 11%, with no difference in rates between IDUs and NIDUs [13]. Gyarmathy and colleagues studied lifetime correlates of HIV, HBV, and HCV infections among a cohort of almost 500 NIDUs in New York City. Overall, 31% tested positive for HBV. Significant correlates of past infection among drug users who had never injected were: unprotected sex with men who have sex with men, history of syphilis infection, and longer duration of heroin use. The authors’ main conclusion regarding NIDUs is that risk for HBV is primarily from unprotected sex [14].
Prevalence of hepatitis C virus (HCV) among IDUs is endemically high, with 65% or more of IDUs infected within the first year of injection, and close to 90% of longer-term injectors infected [15, 16]. Prevalence of HCV among NIDUs is lower than that among IDUs, but still higher than among the general public; this may be due to sharing of pipes and straws for smoking and snorting, high-risk sex [17, 18], and/or misclassification, as many people are reluctant to report injection drug use due to stigma.
HCV-negative drug users may be a valuable subgroup for prevention, and studying them may offer an insight into the relative importance of injection and non-injection routes for the transmission of HBV. We examined HBV prevalence and risk behaviour patterns among a group of HCV-negative drug users in order to understand the risk for HBV and the needs and prevention opportunities among this unique group.
METHODS
Participant recruitment
Between January 2002 and January 2004, participants were recruited for a health service research study of drug users. The study was advertised as a ‘quality of life’ research project with a financial incentive at various community agencies, through newspaper advertisements, and on the street with flyers. Those interested were directed to call the study telephone number to be screened by study research assistants. If eligible after a telephone screening that included demographics, recent drug use, and questions regarding psychosis, individuals were asked to come to the research site at Rhode Island Hospital, Providence, for a more detailed assessment. Inclusion criteria included the following: (1) age between 18 and 70 years; (2) drug injection during the preceding 30 days or heroin or cocaine use at least weekly for the past 6 months; (3) fewer than 30 of the last 90 days spent in institutional settings including prison, residential drug treatment or hospitalization; (4) ability to speak English; (5) denial of intent to harm self or others; and (6) written consent for this study, which was approved by the Rhode Island Hospital/Lifespan Institutional Review Board.
Participants were administered a 90-min structured interview that included sections on demographics, mood, drug and alcohol use, impulsivity, and adverse life events. Individuals participating in the interview received compensation of $20.
Measures
Sociodemographics included age, gender, race/ethnicity, and education. Indicators of substance use were years of heroin use, years of cocaine use, history of ever injecting drugs, history of injecting drugs in the last 6 months, the drug-risk component of the the HIV Risk Assessment Battery (RAB) [9], and number of days using alcohol to intoxication in the past 6 months (subjectively defined as feeling drunk or feeling the effects).
Because we hypothesized that sexual risk-taking contributed to HBV exposure, we tested several measures of sexual risk. Variables regarding sexual activity in the last 6 months included any vaginal or anal sex, number of sexual partners, number of sexual events with non-primary partners, any exchange of sex for drugs or money, and the sexual risk component of the HIV RAB. The RAB scoring protocol generates scale scores from 0 to 1·0 with high scores indicating relatively higher levels of HIV sexual risk [19].
Hepatitis C antibody was tested by enzyme immunoassay (Abbott HCV EIA 2.0, Abbott Laboratories, Abbott Park, IL, USA). Hepatitis B surface antigen, surface antibody, and core antibody were assessed by enzyme immunoassay (AUSZYME Monoclonal, AUSAB EIA, and CORZYME diagnostic kits, Abbott Laboratories). For the purposes of the analyses, a participant was considered exposed to HBV if any one of the three hepatitis B indicators – hepatitis B surface antibody (HBsAb), hepatitis B core antibody (HBcAb), or hepatitis B surface antigen (HBsAg) – was positive. While common practice dictates that positive surface antibody alone is indicative of vaccination [3], not infection, clinical practice, confirmed by studies of IDUs from before the advent of the HBV vaccine, demonstrate that this serological profile is possible even in persons who have not been vaccinated [20]. Given the historically low vaccination coverage amongst IDUs [13], we assumed that sole HBsAb positivity was a result of infection and not vaccination.
Analytical methods
Several measures were markedly skewed and the distributional assumptions of normal theory methods were not well approximated. In addition to means and standard deviations we report medians and the interquartile range (IQR) to describe continuous variables. We used bivariate and multivariate logistic regression to summarize the association between HBV status and selected predictors. In all cases we report the effect of the selected predictor on the expected odds of positive HBV exposure. Continuous variables were standardized to zero mean and unit variance prior to estimating the logistic regression models; the associated effects are sometimes referred to a partially standardized [21] or x-standardized [22]. In the multivariate analyses, our primary interest was to determine if indicators of high-risk sexual behaviours were associated with HBV exposure after controlling for background characteristics and indicators of drug and alcohol use behaviours. The sex-risk indicators exhibited relatively high multicolinearity; therefore, we estimated four separate multivariate logistic regression models. Each model included all demographic characteristics, all indicators of drug and alcohol use behaviours, but only one of the four sex-risk indicators. To keep the presentation concise, we report complete results for only one of the four models. Specifically, the adjusted coefficients we report in Table 1 for background characteristics and substance use indicators were estimated for the model that included only the RAB drug-risk index. The adjusted coefficients we report for the four sex-risk indicators were separately estimated. Readers should note that the effects of background characteristics and substance use indicators were neither substantively nor statistically different across the four models.
Table 1.
Unadjusted and adjusted effects of selected predictors on hepatitis B exposure (n=164)
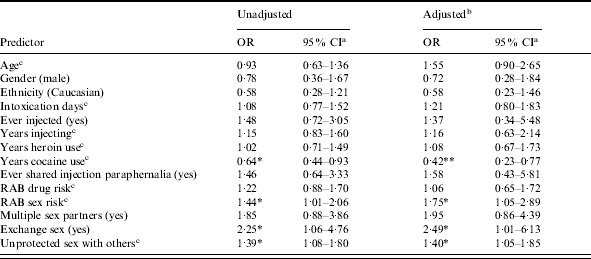
OR, Odds ratio; CI, confidence interval; RAB, Risk Assessment Battery.
Tests of significance and confidence interval estimates use robust standard error estimators.
Adjusted effects for background characteristics and substance use indicators were estimated with RAB sex risk as a covariate.
Continuous variables standardized to zero mean and unit standard deviation prior to estimation. The reported coefficients give the expected factor change in the expected odds of positive hepatitis B exposure for a 1 s.d. increase in the predictor variable.
P<0·05, ** P<0·01.
RESULTS
During the recruitment period, the study telephone received 1390 calls. Of the 344 potentially eligible individuals who scheduled and kept appointments, 14 were deemed ineligible based on a more thorough screening. Serological testing of these 330 drug users identified 164 HCV-antibody negative individuals, who ultimately enrolled in the study.
Participants averaged 37·3 years of age and 11·6 years of education (Table 2). Just over two-thirds (68·3%) were male and 48·8% were Caucasian. Thirty-nine (23·8%) participants tested positive for exposure to HBV; 19 were solely HBsAb positive, 20 were HBcAb positive and one was HBsAg positive. Seventy-two (43·9%) participants had injected drugs. In total, 27·8% of drug injectors tested positive for exposure to HBV compared to 20·7% of those who had never injected drugs (P=0·287). Study participants were not tested for HIV, but 138 participants reported having been tested, and eight reported being HIV seropositive.
Table 2.
Background characteristics and descriptive statistics
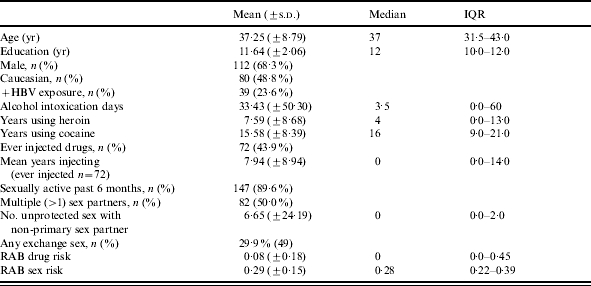
IQR, Interquartile range; RAB, Risk Assessment Battery.
Forty-five (27·4%) participants reported they had never used heroin. Among those who had used heroin, the mean number of years participants reported using heroin was 7·59 years (±8·68; median=4) (Table 2). Only one participant had never used cocaine; among those who did use cocaine, mean years of cocaine use was 15·6 years. Among the 72 participants who had ever injected, the mean time since first drug injection was 7·9 years and 70·8% had injected within the last 6 months. Almost 90% of the participants were sexually active and half (50·0%) reported multiple sex partners in the 6 months prior to interview (Table 2). The number of times participants reported having unprotected sex with non-primary partners was very skewed. While participants reported having unprotected sex with non-primary partners an average of 6·7 (±8·68) times the median was 0 and the IQR was 0–2. Almost 30% said they had engaged in some form of exchange sex (24 said they had sex to obtain drugs, 27 said they had given drugs to someone for sex, 29 said they had sex to obtain money, and 10 said they had paid money for sex), and RAB sex-risk scores averaged 0·29 (Table 2). Only six men identified their sexual orientation as homosexual or bisexual; two (40%) of the men who reported having sex with men had positive HBV exposure, but the statistical power is not sufficient to conduct adequate analyses.
Overall, 22 (13·4%) participants reported they had been vaccinated for HBV, 20 (12·2%) said they did not know if they had ever been vaccinated, and 122 (74·4%) said they had never been vaccinated for HBV. There were 19 participants (11·6%) who tested positive only for HBV surface antibodies; of these, nine (47·4%) said they had been vaccinated for HBV, two (10·5%) said they were not sure if they had ever been vaccinated, and eight (42·2%) said they had not been vaccinated for HBV.
Table 1 gives unadjusted and adjusted effects summarizing the associations between exposure to HBV and selected predictor variables. Testing positive for exposure to HBV was generally associated with indicators of greater sex risk (Table 1). Testing positive for HBV was significantly associated with engaging in exchange sex [odds ratio (OR) 2·25, P=0·035], number of times the participant reported having unprotected sex with someone who was not their primary sex partner (OR 1·39, P=0·011), and with the RAB sex-risk index (OR 1·44, P=0·047). Participants who reported more than one sex-partner tended to be more likely to test positive for exposure to HBV, although this association was not significant at the conventionally accepted 0·05 level (OR 1·85, P=0·102). HBV exposure status was not associated significantly with demographic characteristics such as age, gender, or ethnicity. With the exception of years using cocaine, HBV exposure status was not associated significantly with indicators of drug or alcohol use or injection history. Participants who reported longer duration of cocaine use were significantly less likely to test positive for exposure to HBV (OR 0·64, P=0·019).
We used logistic regression analysis to estimate adjusted effects. Because the indicators of sex risk exhibited relatively high-multicolinearity we estimated four separate logistic regression models; each included all demographic and drug-risk indicators and one sex-risk indicator. Specifically, we estimated in separate models the unique effects of the RAB sex-risk index, exchange sex, unprotected sex with non-primary partners, and having multiple sexual partners. For demographic characteristics, substance use, and drug-risk indicators we report the adjusted parameter estimates only for the model that included the RAB sex-risk index as a predictor (Table 1). These coefficients were of similar magnitude in all four models.
The adjusted effects estimated using multivariate logistic regression are consistent with the unadjusted associations (Table 1). HBV status was not associated with background characteristics such as age, gender, or ethnicity. With the exception of years of cocaine use, none of the drug-risk variables – including whether or not participants reported a history of drug injection – were significant predictors of HBV status. The likelihood of positive HBV exposure was inversely associated with the number of years participants reported using cocaine (OR 0·42, P=0·002) after adjusting for the other covariates included in the model. After adjusting for potential confounders, higher sex risk, as measured by the RAB sex-risk index, was significantly associated with HBV status (OR 1·75, P=0·031). In separate models we also found that any exchange sex (OR 2·49, P=0·048) and frequency of unprotected sex with non-primary partners (OR 1·40, P=0·020) were associated significantly with HBV-positive status. The adjusted association between having multiple sex partners and HBV status was directionally consistent with expectations, although not statistically significant (OR 1·95, P=0·109).
DISCUSSION
In this study of HCV-negative illicit drug users, 24% had evidence of past infection with HBV. Although the long-time drug users in this cohort have somehow avoided HCV, a highly prevalent virus with overlapping routes of transmission, almost one quarter has nevertheless been infected with HBV. By comparison, prevalence of past HBV infection among most cohorts of IDUs is up to three times as high as the prevalence we report here [5–10], so whatever protected participants from HCV probably afforded some similar protection from HBV. Therefore, HCV-negative drug users are indeed at high risk for HBV, but their risk appears not be as high as that of HCV-positive drug users. Further study is necessary to determine whether these protections were behavioural or immunological.
HBV is much more readily transmitted through unprotected sex than is HCV [5, 16]. Our findings – that testing positive for exposure to HBV was generally associated with indicators of greater sex risk – are consistent with the hypothesis that HBV risk among HCV-negative drug users is primarily due to sexual behaviour [14, 23]. Specifically, testing positive for exposure to HBV was generally associated with engaging in exchange sex, number of times participants reported unprotected sex with someone who was not their primary sexual partner, and higher scores on the RAB sex-risk index. These high-risk sexual activities place participants at high risk for HIV infection as well as HBV infection. Prevention efforts, therefore, should include both HBV vaccination and education aimed at reducing high-risk sexual behaviours.
One counterintuitive finding is that participants who reported longer duration of cocaine use were significantly less likely to test positive for exposure to HBV. Given the fact that stimulant use often leads to diminished sexual inhibitions, this finding is, indeed, counterintuitive. Others have reported similar findings, hypothesizing that long-term cocaine users may constitute an insulated community that does not interact with injectors who may be at higher risk [14]. Further study is needed to determine what other socio-biological factors might be at play.
Because of questions about the limited reliability of HBsAb to determine whether participants had been vaccinated, we were able to draw few conclusions about the prevalence of vaccination in this cohort. Self-reported vaccination history did not correlate with serology: of those whose serology was consistent with vaccination, only half reported having been vaccinated, and almost half reported not having been vaccinated. If we assume that a handful of participants with HBsAb alone were not, in fact, vaccinated, the vaccination rate would be even lower than 12%. Although this vaccination rate is in keeping with reported rates among other cohorts of drug users [24], it falls conspicuously short of the stated goals of the Healthy People 2000 objectives: to vaccinate 90% of people at high risk for HBV by 2000 [2].
Limitations of this study include the potential for HCV-positive participants to have been misclassified as HCV-negative [25, 26]. However, even among HIV co-infected persons, false-negative antibody tests are uncommon, and our cohort had very few HIV-positive persons. Further, the cohort was, to some extent, a heterogeneous population, and all behaviours were self-reported. Another limitation is related to the cross-sectional design of the study. Participants were asked about their risk behaviours in the past 6 months, while serum markers for HBV infection may reflect an infection acquired quite some time ago, and behaviours may have changed in intervening years since acquisition.
In conclusion, drug use itself, regardless of route of drug administration, identifies high risk for HBV, and thus, all drug users – whether they inject or not – should be targeted for HBV vaccination. However, whereas risk for HBV among IDUs stems primarily from injection behaviours, risk among NIDUs seems to stem from high-risk sexual behaviour. Further, cocaine use seems to offer some protective effect against HBV, although the mechanisms for this remain unclear. Due to shared routes of transmission, persons at risk for HBV are also at risk for HIV acquisition and transmission. Preventive interventions are needed for both sexual and drug-related high-risk behaviour.
ACKNOWLEDGEMENTS
This work was funded by RO1 DA 13759 and by grant number P30-AI-42853 from the National Institutes of Health, Center for AIDS Research (NIH CFAR). Dr Stein is a recipient of a NIDA Mid-Career Investigator Award.
DECLARATION OF INTEREST
None.
REFERENCES
- 1.Centers for Disease Control and Prevention. Incidence of acute hepatitis B – United States, 1990–2002. Morb Mortal Wkly Rep. 2004;52:1252–1254. [PubMed] [Google Scholar]
- 2.National Center for Health Statistics. Healthy People 2000: National Health Promotion and Disease Prevention Objectives. Atlanta: 1990. : CDC, [Google Scholar]
- 3.Centers for Disease Control and Prevention. Hepatitis B virus: a comprehensive strategy for eliminating transmission in the United States through universal childhood vaccination: recommendations of the Immunization Practices Advisory Committee (ACIP) Morb Mortal Wkly Rep. 1991;40:1–19. [PubMed] [Google Scholar]
- 4.Goldstein ST, Alter MJ, Williams IT et al. Incidence and risk factors for acute hepatitis B in the United States, 1982–1998: implications for vaccination programs. J Infect Dis. 2002;185:713–719. doi: 10.1086/339192. [DOI] [PubMed] [Google Scholar]
- 5.Centers for Disease Control and Prevention. The pink book: epidemiology and prevention of vaccine-preventable diseases. Atlanta: Centers for Disease Control and Prevention. 6th edn. 2001. Hepatitis B. pp. 207–229. [Google Scholar]
- 6.Lopez-Zetina J, Kerndt P, Ford W et al. Prevalence of HIV and hepatitis B and self-reported injection risk behavior during detention among street-recruited injection drug users in Los Angeles County, 1994–1996. Addiction. 2001;96:589–595. doi: 10.1080/09652140020031638. [DOI] [PubMed] [Google Scholar]
- 7.Tortu S, McMahon JM, Hamid R et al. Women’s drug injection practices in East Harlem: an event analysis in a high-risk community. AIDS Behav. 2003;7:317–328. doi: 10.1023/a:1025452021307. [DOI] [PubMed] [Google Scholar]
- 8.Samuel MC, Doherty PM, Bulterys M et al. Association between heroin use, needle sharing and tattoos received in prison with hepatitis B and C positivity among street-recruited injecting drug users in New Mexico, USA. Epidemiol Infect. 2001;127:475–484. doi: 10.1017/s0950268801006197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Novick DM, Gelb AM, Stenger RJ et al. Hepatitis B serologic studies in narcotic users with chronic liver disease. Am J Gastroenterol. 1981;75:111–115. [PubMed] [Google Scholar]
- 10.Levine OS, Vlahov D, Nelson KE. Epidemiology of hepatitis B virus infections among injecting drug users: seroprevalence, risk factors, and viral interactions. Epidemiol Rev. 1994;12:418–436. doi: 10.1093/oxfordjournals.epirev.a036161. [DOI] [PubMed] [Google Scholar]
- 11.Substance Abuse and Mental Health Services Administration. 2003. www.drugabusestatistics.samhsa.gov. www.drugabusestatistics.samhsa.gov The NHSDA Report: Injection drug use. Office of Applied Studies, Substance Abuse and Mental Health Services Administration. ). Accessed 30 August 2005.
- 12.Friedman SR, Flom PL, Kottiri BJ et al. Drug use patterns and infection with sexually transmissible agents among young adults in a high-risk neighbourhood in New York City. Addiction. 2003;98:159–169. doi: 10.1046/j.1360-0443.2003.00271.x. [DOI] [PubMed] [Google Scholar]
- 13.Kuo I, Sherman SG, Thomas DL et al. Hepatitis B virus infection and vaccination among young injection and non-injection drug users: missed opportunities to prevent infection. Drug Alc Depend. 2004;73:69–78. doi: 10.1016/j.drugalcdep.2003.10.007. [DOI] [PubMed] [Google Scholar]
- 14.Gyarmathy VA, Neaigus A, Miller M et al. Risk correlated of prevalence HIV, hepatitis B virus, and hepatitis C virus infections among noninjecting heroin users. J Acquir Immune Defic Syndr. 2002;30:448–456. doi: 10.1097/00042560-200208010-00011. [DOI] [PubMed] [Google Scholar]
- 15.Garfein RS, Vlahov D, Galai N et al. Viral infections in short-term injection drug users: the prevalence of the hepatitis C, hepatitis B, human immunodeficiency, and human T-lymphotrophic viruses. Am J Pub Health. 1996;86:655–661. doi: 10.2105/ajph.86.5.655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Alter MJ. Epidemiology of hepatitis C. Hepatology. 1997;26:62S–65S. doi: 10.1002/hep.510260711. [DOI] [PubMed] [Google Scholar]
- 17.Tortu S, McMahon JM, Pouget ER, Hamid R. Sharing of noninjection drug-use implements as a risk factor for hepatitis C. Substance Use Misuse. 2004;39:211–224. doi: 10.1081/ja-120028488. [DOI] [PubMed] [Google Scholar]
- 18.McMahon JM, Tortu S. A potential hidden source of hepatitis C infection among noninjecting drug users. J Psychoactive Drugs. 2003;35:455–460. doi: 10.1080/02791072.2003.10400492. [DOI] [PubMed] [Google Scholar]
- 19.Navaline HA, Snider EC, Petro CJ et al. An automated version of the Risk assessment battery (RAB): enhancing the assessment of risk behaviors. AIDS Res and Human Retroviruses. 1994;10:S281–S283. [PubMed] [Google Scholar]
- 20.Novick DM, Gelb AM, Stenger RJ et al. Hepatitis B serologic studies in narcotic users with chronic liver disease. Am J Gastroenterol. 1981;75:111–115. [PubMed] [Google Scholar]
- 21.Menard S. Applied logistic regression analysis. Sage University Paper Series on Quantitative Applications in the Social Sciences, 07-106. Thousand Oaks, CA: 1995. [Google Scholar]
- 22.Long JS. Regression models for categorical and limited dependent variables. Thousand Oaks, CA: Sage; 1997. [Google Scholar]
- 23.Blanck RR, Ream N, Conrad M. Hepatitis B antigen and antibody in heroin users. Am J Gastroenterol. 1979;71:164–167. [PubMed] [Google Scholar]
- 24.Thiede H, Hagan H, Murrill CS. Methadone treatment and HIV and hepatitis B and C risk reduction among injectors in the Seattle area. J Urban Health. 2000;77:331–345. doi: 10.1007/BF02386744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chamot E, Hirschel B, Wintsch J et al. Loss of antibodies against hepatitis C virus in HIV-seropositive intravenous drug users. AIDS. 1990;4:1275–1277. doi: 10.1097/00002030-199012000-00016. [DOI] [PubMed] [Google Scholar]
- 26.Bonacini M, Lin HJ, Hollinger FB. Effect of coexisting HIV-1 infection on the diagnosis and evaluation of hepatitis C virus. J Acquir Immune Defic Syndr. 2001;26:340–344. doi: 10.1097/00126334-200104010-00008. [DOI] [PubMed] [Google Scholar]


