SUMMARY
During a period of 3 years, 1998–2000, 1047 faecal swabs from Black-headed gulls were sampled at one location in Southern Sweden. Salmonella spp. was found in 28 individuals (2·7%) and the dominating serotype found was S. Typhimurium (83%). Twenty-five per cent of the Salmonella-infected gulls were later recaptured and re-sampled. We found that Salmonella infection in Black-headed gulls was of short duration, and that infection in this bird species was predominantly expressed as carriage without disease manifestations. All S. Typhimurium isolates were subjected to antibiotic resistance profiling and molecular characterization by pulsed-field gel electrophoresis and IS200 polymerase chain reaction. The S. Typhimurium gull isolates were compared to human and domestic animal isolates of the same serotype and phage type. We found genetic relatedness of S. Typhimurium DT195 isolates from gulls, domestic animals and humans, indicating that Black-headed gulls might play a role in the spread of S. Typhimurium in Sweden.
INTRODUCTION
Salmonella is a zoonosis with a number of wild and domestic animal hosts [1–6]. There are more than 2500 serovars or serotypes of this bacterium known today. Some serotypes have developed host adaptation and are, therefore, found in only a few animal reservoirs [7–11]. However, most serotypes are ubiquitous or unadapted and can be found in a number of animal hosts.
As a result of legislation and strict control measures applied to the domestic animal food production chain, Sweden has less Salmonella-infected domestic animals than the majority of European countries [6]. Human salmonellosis in Sweden is dominated by cases associated with travelling abroad (∼85% of reported clinical cases). Domestic and non-domestic cases are dominated by two Salmonella serotypes S. Typhimurium and S. Enteritidis. S. Typhimurium is found in a number of animal reservoirs, while S. Enteritidis is often associated with poultry and other fowl [12–16].
The role of wild animals in Salmonella epidemiology is debated. A survey of wildlife was performed in Sweden in 1999, including deer, hares, moose, wild boars, Canada geese and gulls. Of these only gulls yielded positive Salmonella samples [17]. Studies focusing on wild birds as carriers of Salmonella have shown that bird species which live close to humans and feed on human garbage or sewage are more likely to carry Salmonella and that carriage in the wild bird population is low [18–21]. Wild birds spreading enteropathogenic bacteria to the food and shelters of domestic animals, or food-processing plants like dairies are suggested as a risk factor for human and domestic animal salmonellosis [22–27]. Previous studies have pointed to gulls, especially the Black-headed gull (Larus ridibundus), as the most important wild bird Salmonella reservoir in Europe [21, 23, 28–30], and to S. Typhimurium as the most common serotype found in wild birds [28, 29].
The aim of this study was to further investigate the role of Black-headed gulls as carriers of Salmonella and their impact on Salmonella epidemiology. We report on the prevalence of Salmonella in Black-headed gulls of different ages and populations, captured at one site in Sweden. Using re-capture and re-sampling, we had a unique opportunity to study Salmonella carriage longitudinally in the Black-headed gull population.
MATERIAL AND METHODS
Sampling strategy
During a period of 3 years, 1998–2000, a total of 1047 faecal samples were collected from Black-headed gulls. All gulls, excluding nestlings, were caught in a central park, in Malmö, southern Sweden (Table 1). The birds were captured in cage-like traps, identified as to species, age-determined and ringed. Faecal samples were collected by inserting a cotton-coated swab in the cloak, and transported to the laboratory in charcoal transport media (Transwab, BioDisc, Solna, Sweden). Each faecal sample was marked with the corresponding ring number. When Salmonella-positive gulls were re-captured a new sample was collected. Information on the movements of individual gulls was obtained from the Swedish Bird Ringing Centre, Swedish Museum of Natural History. The Swedish Bird Ringing Centre collects reports of ornithologists and the public on ringed birds, either by ringing reports, or by observation or capture of ringed birds.
Table 1.
Total number, serovars and S. Typhimurium phage types (DT) in Black-headed gulls sampled in Malmö, Sweden, 1998–2000
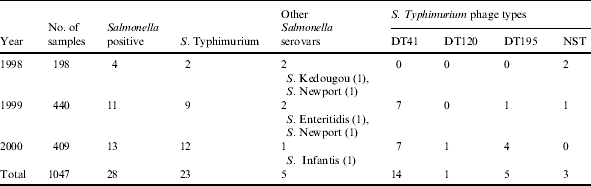
NST, Non-serotypable.
To be able to look into seasonality of Salmonella carriage in Black-headed gulls, the samples were divided into five seasonal groups. In January–February samples were taken from stationary birds, in March–April from spring-migrating birds, in June from nestlings, in July–August from juvenile birds and in October–November from autumn-migrating birds (Table 2). In 1998, only spring-migrating birds were sampled. In 1999 all groups, excluding nestlings, were sampled, while in 2000 all groups were represented. In total, 198 birds were sampled in 1998, 440 in 1999 and 409 in 2000. Thirty nestlings were sampled in a colony in central Malmö in June 2000.
Table 2.
Number of sampled Black-headed gulls according to season and age
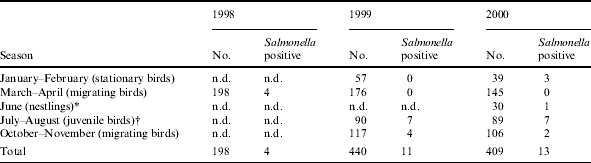
n.d., Not done.
Only nestlings were sampled and ringed in June.
All captured birds in July–August were juvenile birds. The adult birds do not reside in the capture area at this time of the year.
Isolation and identification of Salmonella
Sampling and re-sampling were done according to the same procedure. Our aim was to identify human associated Salmonella, therefore, the analysing method used was the recommended procedure of a Swedish accredited laboratory for clinical human medicine. The bacteriological analyses were performed 1–3 days after sampling. Samples were sent to the laboratory by mail, or otherwise stored at 4–8°C until analysed. Each sample was enriched in selenite broth (Oxoid AB, Stockholm, Sweden), incubated at 37°C for 18–24 h, and subcultured on xylose-lysine-desoxycholate agar. Black H2S precipitating colonies were verified as Salmonella by their reaction in fermentation tests and with a Salmonella polyvalent phage FO-1 (Reagensia AB, Stockholm, Sweden). Isolates were then sent for serotyping and phage typing to the Swedish Institute of Infectious Disease Control [31]. Serotyping was carried out according to the Kaufmann–White serotyping scheme [32]. Phage typing of S. Typhimurium was done according to Anderson [33].
Characterization of isolated Salmonella strains
All Salmonella isolates collected from Black-headed gulls were characterized by pulsed-field gel electrophoresis (PFGE), IS200 polymerase chain reaction (PCR) and antibiotic resistance typing. In the two former investigations, isolates collected from domestic animals and human clinical cases were included for comparison. PFGE was performed according to Palmgren [34] using three restriction enzymes, SpeI, BlnI and XbaI (Boerhinger–Mannheim, GmbH, Germany). IS200 typing was done by PCR amplification of fragments located between the IS200 elements, as described by Millemann [35]. Antibiotic resistance to sulfisoxazole, streptomycin, nalidixic acid, ciprofloxacin, gentamycin, ampicillin, trimethoprim, and chloramphenicol was determined by the disk diffusion method with paper disks on PDM agar (AB Biodisk, Solna, Sweden), according to instructions from the Swedish Reference Group for Antibiotic and Resistance Methods (RAF-M; http://ltkronoberg.se/ext/raf/raf.htm). The zone of growth inhibition was measured with calipers and classified as sensitive, intermediate or resistant using the break-points predetermined after standardization by RAF-M.
Strains used for comparative studies by PFGE and IS200 typing
Fifty-four S. Typhimurium DT (definitive type) 41 isolates originating from human clinical cases (n=31) and domestic animals (n=23), as well as 13 isolates of S. Typhimurium DT195, nine human and four from domestic animals, were included for comparison. All isolates used in the study were from the three study years 1998–2000.
Human isolates were recovered from the Swedish Institute of Infectious Disease Control where the reference laboratory for serotyping and phage typing of Salmonella is situated. The Salmonella isolates from domestic animals came from the National Veterinary Institute. All isolates in the study were serotyped and phage typed according to the same methods and all phage typing was done at the same laboratory at the Swedish Institute of Infectious Disease Control.
S. Typhimurium DT41 isolates
Human
All 31 human DT41 isolates came from clinical cases of salmonellosis or Salmonella carriage. Twenty-four isolates came from presumed domestic cases, while four patients had a previous history of travel outside Sweden (Table 3). For three of the isolates no epidemiological information as to country of origin was stated.
Table 3.
PFGE type and IS200 type of isolates of S. Typhimurium DT41 and DT195 found in Sweden 1998–2000
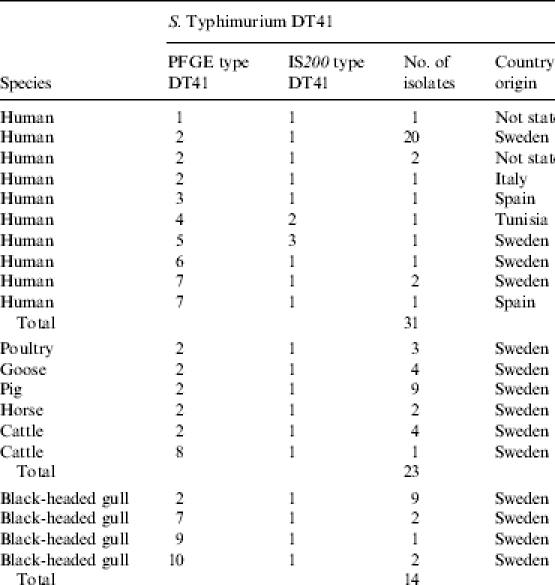
Human isolates with different country of origin via the Swedish Institute for Infectious Disease Control (SMI). Domestic animal isolates via the National Veterinary Institute (SVA) and the Black-headed gull isolates from this study. 43 S. Typhimurium DT41 and 18 S. Typhimurium DT195 were analysed with both typing methods.
Domestic animals
The 23 isolates from domestic animals all had Sweden as country of origin. They were divided according to animal species (Table 3). Isolates came from poultry (3), goose (4), pig (9), cattle (5) and horse (2).
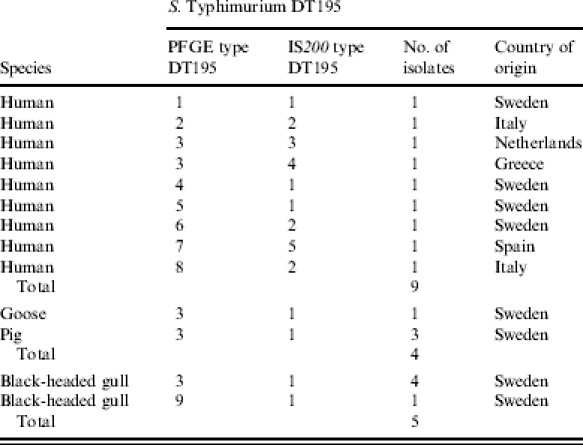
S. Typhimurium DT195 isolates
Human
Nine isolates from human clinical cases or carriers were divided according to country of origin. Four isolates were considered domestic while five isolates came from patients with a history of travelling abroad prior to infection (Table 3).
Domestic animals
Isolates from a Swedish goose and three pigs were also included in the epidemiological study.
RESULTS
Of the 1047 Black-headed gulls sampled in 1998, 1999 and 2000, 28 (2·7%) individuals were Salmonella positive (Table 1). Twenty-four of the 28 Salmonella isolates were collected in 1999 and 2000, in Black-headed gulls sampled at different seasons of the year (Table 2). Fourteen of these 24 isolates (58%) were found in juvenile gulls born in Sweden (Table 2). Salmonella carriage in juvenile birds was significantly higher (7·8%, P<0·001, χ2 test) than in adult birds. All the Salmonella-positive juvenile birds were captured in the summer period July–August when they constitute the vast majority of Black-headed gulls at the capture site in Malmö. During this part of summer, when the nests are empty, the adult birds are found at sea and are not captured in the city area. In 2000, one nestling (3·3%) was found to be Salmonella positive. The remaining 13 Salmonella-positive samples were found in birds considered as adult birds, aged ⩾1 year. A total of 2% (4/198 samples) of the birds sampled in the spring migration period were Salmonella positive while 3·1% (3/96) of the stationary birds sampled in the winter of 2000 and 2·7% (6/223) of the autumn-migrating birds were Salmonella positive.
Out of the 28 isolates retrieved in the whole study, 23 were identified as S. Typhimurium. Fourteen of these belonged to DT41, five to DT195, one to DT120 and three isolates were non-serotypable (NST). Furthermore, two isolates were identified by serotyping as S. Newport and one each as S. Enteritidis, S. Infantis and S. Kedougou (Table 1).
All of the Salmonella-positive birds appeared to be in good health at the time of the Salmonella-positive sampling. Six (25%) of the 24 birds found positive for Salmonella in 1999 and 2000 were sampled at least twice. None of these birds was found to be positive on more than at one occasion. Two of them had been sampled previously, and are, therefore, known to have been Salmonella negative at 8 and 63 days respectively, before being sampled and found to be Salmonella positive. Four birds were re-sampled as Salmonella negative at days 13, 18, 71 and 119 respectively, after being Salmonella positive.
History of recaptured birds
A juvenile Black-headed gull born in Malmö in May 2000 was sampled in August, and was found to be positive for Salmonella. The bird was later re-captured and sampled on two occasions, once in October and once in November. On both these occasions it was found to be Salmonella negative and apparently healthy. Two weeks after the third sampling, the bird was found dead in a city park in Malmö. No apparent cause of death could be determined on inspection. The bird carcass was not available for sampling for cultivation.
Another individual was sampled as an adult, during the sampling session of stationary birds in February 2000, and found to be Salmonella positive. This bird was subsequently re-sampled and found to be negative on three occasions: 3 weeks, 2 months and 9 months after the positive sampling.
Molecular characterization of isolates
S. Typhimurium isolates from Black-headed gulls, of DT41 (n=14) and DT195 (n=5), were subjected to further characterization by PFGE and IS200 typing. The S. Typhimurium DT41 isolates were compared to isolates of the corresponding phage type collected from domestic animals (n=25) and human clinical cases (n=31) between 1997 and 2000.
By IS200 PCR, three different banding patterns were found (IS200 types 1–3, Table 3). All S. Typhimurium DT41 isolates of gull and animal origin and the majority of isolates from humans were found to be identical and belonged to IS200 type 1. Only two human isolates differed in banding patterns (Table 3).
PFGE analysis, however, showed a number of minor differences between isolates, resulting in 10 PFGE subtypes, denoted PFGE type DT41 1–10 (Table 3). The vast majority of the isolates from gulls, domestic animals and human clinical cases, 57 out of 70 analysed, were of PFGE type 2. Some PFGE types, namely types 1 (n=1), 3 (n=1), 4 (n=1), 5 (n=1) and 6 (n=1), were unique to human isolates. PFGE types 9 (n=1) and 10 (n=2) were found in isolates from Black-headed gulls only and PFGE type 8 (n=1) was unique for a Salmonella isolate found in cattle, whereas PFGE type 7 was found both among human (n=3) and gull (n=2) isolates.
Furthermore, gull isolates of S. Typhimurium DT195 were compared to DT195 isolates collected between 1997 and 2000 from domestic animals (n=4) and human clinical cases (n=9). IS200 PCR typing resulted in five different IS200 types. Using PFGE typing, seven subtypes could be identified. All domestic animal and gull isolates were identical with both methods. With IS200 PCR typing, three of the human isolates were grouped with the isolates of animal origin, but by PFGE only one of these isolates showed complete identity with the animal isolates.
Antibiogram
All 28 Salmonella isolates were tested for antibiotic resistance by the disk diffusion method. Multiresistance, defined as antibiotic resistance against three or more antibiotics, was found in two isolates of serotypes Newport and Typhimurium NST, both collected during the spring migration in 1998. One of these was resistant to sulfisoxazole, ampicillin and trimethoprim, the other to sulfisoxazole, ampicillin, streptomycin and cloramphenicol. No other antibiotic resistance was observed.
DISCUSSION
From a total of 1047 Black-headed gulls, caught at one location in Sweden between 1998 and 2000, 28 Salmonella spp. (2·7%) were isolated. The dominating serotype was S. Typhimurium (83%), the other serotypes found being S. Enteritidis, S. Infantis, S. Kedougou and S. Newport. Based on results of a study performed in 1997 [29], the sampling focused on Black-headed gulls in 1998, 1999 and 2000. In 1999, we introduced a strategy of sampling and re-sampling of Black-headed gulls, that allowed us to, under natural conditions, study the duration of Salmonella carriage in Black-headed gulls and determine if positive birds showed any signs of disease. By registering the faecal sample together with the ring number of each gull, we could get further information on individual Salmonella-positive birds, either by re-sampling the bird or by ornithologists’ observations.
Twenty-five per cent of the Salmonella-positive gulls were sampled at least twice. There were no signs of morbidity or mortality in Salmonella-positive gulls, and we had reports on three occasions that previously Salmonella-positive gulls had completed their migrations to Denmark and Holland respectively.
Girdwood and colleagues found the average duration of Salmonella excretion in a related species, the Herring gull (Larus argentatus), to be 2 days with a maximum duration of 4 days, in a challenge experiment with caged birds [36]. Our study in Black-headed gulls support their observations that Salmonella does not belong to the normal enteric bacterial flora of the gulls and the duration of Salmonella carriage is relatively short. This is also supported by the fact that none of the gulls were Salmonella positive on more than one sampling occasion, even if there was a short period between the time when the positive sample was taken and the re-sampling. Furthermore, the low incidence of Salmonella in adult Black-headed gulls is another observation pointing in the same direction.
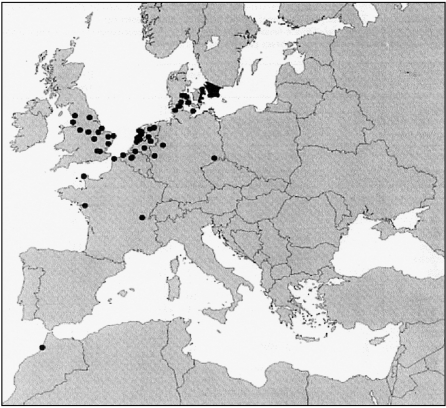
Even if Black-headed gulls are present in the study area throughout the year, ringing recoveries have shown that several different populations can be found in the area [37]. Some individuals are stationary, spending the entire year in the region. Another sub-population breeds in Malmö and spends the winter in Western Europe. Still other birds breed further to the northeast, mainly in southeast Sweden, Finland, the Baltic states, Belarus and in parts of Russia, and either remain in the Malmö area during winter, or just stop over on their migration southwest in autumn and northeast in spring (Fig.). It is, therefore, important to note that the population of Black-headed gulls sampled by us is not uniform. Seasonal differences in Salmonella carriage were examined by sampling Black-headed gulls throughout the year, and dividing the gulls into four groups, spring-migrating birds, juveniles, autumn-migrating birds, and birds stationary in Sweden over the winter period.
Fig.
Recoveries of Black-headed gulls (Larus ridibundus) hatched and ringed in Malmö, Sweden.
We found that Black-headed gulls seem to be unaffected by Salmonella carriage. This is consistent with earlier studies on Herring gulls [36, 38]. The Salmonella-infected gulls in our study have shown no sign of disease and have been able to migrate to their winter quarters, flying long distances, after testing Salmonella positive.
Like many European countries, Sweden has had outbreaks of the multiresistant S. Typhimurium DT104. Other Salmonella strains isolated in Sweden show very little antibiotic resistance [6]. Wild birds flying over long distances in a short time can bring more resistant Salmonella strains into Sweden, as they can be carriers of strains from southern parts of Europe were antibiotic-resistant Salmonella in domestic animals is a larger problem. Black-headed gulls usually winter in Denmark, Germany, Holland, England, Belgium, France and have been found as far south as North Africa [39] (Fig.). During winter, they often congregate in large flocks in estuaries, wetlands and in farmlands. Two multiresistant Salmonella isolates were found in spring-migrating birds in 1998, while the other 26 Salmonella isolates were either antibiotic sensitive or resistant to sulfisoxazole only. This is consistent with a previous study of migrating Black-headed gulls in the same area, where multiresistant Salmonella isolates were found in the spring-migrating gull population [29].
However, our hypothesis that birds migrating to Sweden from their wintering areas, where Salmonella is more commonly found both in human and domestic animal populations should carry more Salmonella than birds born or stationary in Sweden was not confirmed. In 1999 and 2000, we found no Salmonella in spring-migrating birds, while there were Salmonella-positive birds in all the other groups (nestlings, juveniles, autumn-migrating and stationary) (Table 2). Different feeding habits and an immature immune defence are probable explanations to the higher level of Salmonella carriage in juvenile birds compared to adult birds. This pattern has been described previously in studies of Herring gulls and Black-headed gulls [28, 38].
Of the domestic human cases of salmonelloses reported in Sweden, S. Typhimurium DT40 and DT41 are generally considered to be contracted within Sweden, and to have domestic sources. S. Typhimurium DT40 is known to be the cause of the small bird epizootics that from time to time severely affect passerine birds in Sweden and several other countries [40–46]. Where phage typing has been performed, the causative agent has been S. Typhimurium DT40 [46], except for the small bird epizootic in Sweden in the winter of 1998–1999, where a mix of DT40 and the newly described phage type U277 was responsible [40]. This outbreak spread in the small bird population throughout Sweden, in domestic cats and resulted in a few human cases as well [40]. During the same time period, we found no S. Typhimurium DT40 in the gull population. Subtyping by PFGE of the three isolates of S. Typhimurium NST found among the Black-headed gulls, showed no similarity between these isolates and the S. Typhimurium U277 found in the epizootic of small birds and cats. The isolates from gulls also had diverse PFGE patterns among them. The fact that we observed no sign of the 1998–1999 passerine bird epizootic in the Salmonella found in this study is consistent with what we know of the feeding habits of Black-headed gulls [47]. Even if it is omnivorous it is considered a very sporadic event for Black-headed gulls to prey on other birds.
As for S. Typhimurium DT41, the endemic source for human salmonellosis has been more difficult to pinpoint. As this was the most commonly found Salmonella in Black-headed gulls, it would probably have the highest impact on human and domestic animal Salmonella infection. In an attempt to study the possible relation between isolates of domestic animal (n=25) and human (n=31) origin, collected between 1997 and 2000, subtyping of the isolates was performed and compared to the gull isolates by the two methods described as the most discriminatory for S. Typhimurium in the literature, PFGE and IS200 typing [48, 49]. However, only minor differences could be detected between the isolates analysed (Table 3). This indicates a close relationship between isolates of S. Typhimurium DT41, which makes it difficult to draw conclusions as to the importance of Black-headed gulls in the epidemiology of S. Typhimurium DT41.
Isolates of S. Typhimurium DT195 showed greater variability when subtyped by the same methods as the S. Typhimurium DT41 isolates (Table 3). Of five gull isolates, four domestic animal isolates and nine isolates from human clinical cases contracted both abroad and within Sweden, five different types were found by IS200 PCR typing and seven by PFGE typing using three restriction enzymes. All domestic animal and gull isolates were identical with both methods. With IS200 PCR typing, three of the human strains were grouped with the isolates of gull and domestic animal origin. These three strains (2190/98, 3040/99 and 3199/99) were all from human clinical cases, which stated that they had contracted the infection within Sweden. By PFGE, only one of these strains showed complete identity with the animal isolates, and this particular strain (3199/99) was isolated from a person living in Lund, a city close to Malmö, where the Black-headed gulls were sampled.
Salmonella carriage in Black-headed gulls mirrors to some extent the Salmonella burden in the environment but its role in the epidemiology of Salmonella infection in Sweden is not clear. An interesting point is that we only found one gull infected with S. Enteritidis, although this is the second most common serotype of Salmonella found in domestic human cases in Sweden. S. Enteritidis is almost as common in human cases as S. Typhimurium [31], while in Black-headed gulls S. Typhimurium constituted 82% of the Salmonella found and S. Enteritidis only 3·6%. This was an even more surprising finding considering that S. Enteritidis is a serotype with its main reservoir in birds.
Even if Black-headed gulls are short-term carriers of Salmonella, they may be a possible link in Salmonella epidemiology, serving as components for direct or indirect spread to domestic animals and humans. The fact that they seem to be unaffected by their Salmonella carriage makes this even more possible as they seem to be able to migrate in spite of being infected.
In this study we could not distinguish a clear connection between human, domestic animal and Black-headed gull isolates in Sweden although there are some indications of transmission between the species. The possibility still remains that Black-headed gulls have the potential to pick up and transmit Salmonella serotypes or Salmonella with antibiotic resistance that are otherwise uncommon in Sweden due to strict control measures on food, feed and imported domestic animals.
ACKNOWLEDGEMENTS
We thank Anja Heino and Griselda Loreto Palma for excellent technical assistance, Sven Splittorff for catching and sampling of gulls, and Dr Paul Haemig for valuable help with the manuscript. This work was supported by the Centre for Environmental Research, the Medical Faculty of Umeå University, the Swedish Society of Medicine and The Swedish Research Council for Environment, Agricultural Sciences and Spatial Planning FORMAS (2003-1146).
DECLARATION OF INTEREST
None.
REFERENCES
- 1.Okoh AEJ, Onazi M. Notes on Salmonellae isolated from wildlife in Kano zoological gardens. J Wildl Dis. 1980;16:7–10. doi: 10.7589/0090-3558-16.1.7. [DOI] [PubMed] [Google Scholar]
- 2.Minette HP. Epidemiological aspects of salmonellosis in reptiles, amphibians, mollusks and crustaceans – a review. Int J Zoon. 1984;11:95–104. [PubMed] [Google Scholar]
- 3.Wray C, Todd N, McLaren I, Beedell Y, Rowe B. The epidemiology of Salmonella infection of calves: the role of dealers. Epidemiol Infect. 1990;105:295–305. doi: 10.1017/s0950268800047890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wernery U. The prevalence of Salmonella infections in camels (Camelus dromedarius) in the United Arab Emitrates. Br Vet J. 1992;148:445–450. doi: 10.1016/0007-1935(92)90031-U. [DOI] [PubMed] [Google Scholar]
- 5.Malmqvist M, Jacobsson K-G, Häggblom P, Cerenius F, Sjöland L, Gunnarsson A. Salmonella isolated from animals and feedstuffs in Sweden during 1988–1992. Acta Vet Scand. 1995;36:21–39. doi: 10.1186/BF03547700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Anon. Zoonoses in Sweden up to and including 1999. Reklam och Katalogtryck; Uppsala. Sweden: 2001. [Google Scholar]
- 7.Craven SE, Cox NA, Bailey JS, Blackenship LC. Binding of Salmonella strains to immobilized intestinal mucosal preparation from broiler chickens. Avian Dis. 1992;36:296–303. [PubMed] [Google Scholar]
- 8.Pascopella L, Raupach B, Ghori N, Monack D, Falkow S, Small PLC. Host restriction phenotypes of Salmonella typhi and Salmonella gallinarium. Infect Immun. 1995;63:4329–4335. doi: 10.1128/iai.63.11.4329-4335.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bäumler AJ, Tsolis RM, Ficht TA, Adams LG. Evolution of host adaptation in Salmonella enterica. Infect Immun. 1998;66:4579–4587. doi: 10.1128/iai.66.10.4579-4587.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Steinbach G, Lauterbach L, Methner U. Studies of the phenomenon of host adaptation in Salmonella. J Vet Med B. 2000;47:707–719. doi: 10.1046/j.1439-0450.2000.00403.x. [DOI] [PubMed] [Google Scholar]
- 11.Uzzau S, Brown DJ, Wallis T et al. Host adapted serotypes of Salmonella enterica. Epidemiol Infect. 2000;125:229–255. doi: 10.1017/s0950268899004379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.St. Louis ME, Morse DL, Potter ME, DeMelfi TM, Guzewich JJ, Tauxe RV, Blake PA. The emergence of grade A eggs as a major source of Salmonella enteritidis infections. J Am Med Assoc. 1988;259:2103–2107. [PubMed] [Google Scholar]
- 13.Rodrigue DC, Tauxe RV, Rowe B. International increase in Salmonella enteritidis: a new pandemic. Epidemiol Infect. 1990;105:21–27. doi: 10.1017/s0950268800047609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.van de Giessen AW, Dufrenne JB, Ritmeester WS, Berkers PATA, van Leeuwen WJ, Notermans HW. The identification of Salmonella enteritidis poultry flocks associated with an outbreak of human salmonellosis. Epidemiol Infect. 1992;109:405–411. doi: 10.1017/s0950268800050391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Le Bacq F, Louwagie B, Verhaegen J. Salmonella typhimurium and Salmonella enteritidis: changing epidemiology from 1973–1992. Europ J Epidemiol. 1994;10:367–371. doi: 10.1007/BF01719658. [DOI] [PubMed] [Google Scholar]
- 16.Guard-Petter J. The chicken, the egg and Salmonella enteritidis. Environ Microbiol. 2001;3:421–430. doi: 10.1046/j.1462-2920.2001.00213.x. [DOI] [PubMed] [Google Scholar]
- 17.Anon. 2000. http://www.vetinst.dk. http://www.vetinst.dk Dansk Zoonoscenter, Annual Report. ). Accessed May 2001.
- 18.Williams BM, Richards DW, Lewis J. Salmonella infection in the herring gull (Larus argentatus) J Vet Rec. 1976;98:5. doi: 10.1136/vr.98.3.51. [DOI] [PubMed] [Google Scholar]
- 19.Kapperud G, Rosef O. Avian wildlife reservoir of Campylobacter fetus subsp. jejuni, Yersinia spp and Salmonella spp in Norway. Appl Environ Microbiol. 1983;45:375–380. doi: 10.1128/aem.45.2.375-380.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cizek A, Literak I, Hejlicek K, Treml F, Smola J. Salmonella contamination of the environment and its incidence in wild birds. Zentralbl Veterinarmed B. 1994;41:320–327. doi: 10.1111/j.1439-0450.1994.tb00234.x. [DOI] [PubMed] [Google Scholar]
- 21.Hernadez J, Bonnedahl J, Waldenström J, Palmgren H, Olsen B. Salmonella in birds migrating through Sweden. Emerg Infect Dis. 2003;9:753–755. doi: 10.3201/eid0906.030072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Reilly WJ. Human and animal salmonellois in Scotland associated with environmental contamination, 1973–79. Vet Rec. 1981;108:553–555. doi: 10.1136/vr.108.26.553. [DOI] [PubMed] [Google Scholar]
- 23.Coulson JC, Butterfield J, Thomas C. The herring gull Larus argentatus as a likely transmitting agent of Salmonella montevideo to sheep and cattle. J Hyg (Camb) 1983;91:437–443. doi: 10.1017/s0022172400060472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Baird-Parker AC. Foodborne salmonellosis. Lancet. 1990;336:1231–1234. doi: 10.1016/0140-6736(90)92844-8. [DOI] [PubMed] [Google Scholar]
- 25.Davies R, Breslin M. Environmental contamination and detection of Salmonella enterica serovar enteritidis in laying flocks. Vet Rec. 2001;149:699–704. [PubMed] [Google Scholar]
- 26.Kirk JH, Holmberg CA, Jeffrey JS. Prevalence of Salmonella spp. in selected birds captured on California dairies. J Am Vet Med Assoc. 2002;220:359–362. doi: 10.2460/javma.2002.220.359. [DOI] [PubMed] [Google Scholar]
- 27.Koplan JP. Contaminated roof-collected rainwater as a possible cause of an outbreak of salmonellosis. J Hyg (Camb) 1978;81:303–309. doi: 10.1017/s0022172400025146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hubálek Z, Sixl W, Mikulásková M et al. Salmonella in gulls and other free-living birds in the Czech republic. Cent Eur J Public Health. 1995;3:21–24. [PubMed] [Google Scholar]
- 29.Palmgren H, Sellin M, Bergström S, Olsen B. Enteropathogenic bacteria in migrating birds arriving in Sweden. Scand J Infect Dis. 1997;29:565–568. doi: 10.3109/00365549709035895. [DOI] [PubMed] [Google Scholar]
- 30.Hatch JJ. Threats to public health from gulls (Laridae) Int J Environ Health Res. 1996;6:5–16. [Google Scholar]
- 31.Swedish Institute for Infectious Disease Control. 2002. www.smittskyddsinstitutet.se. www.smittskyddsinstitutet.se ). Accessed May 2003.
- 32.Kauffmann F. Serological diagnosis of Salmonella species. Kauffmann-White-Schema Scandinavian University books; Denmark, Munksgaard Copenhagen: 1972. [Google Scholar]
- 33.Anderson ES, Ward LR, Saxe MJ, de Sa JD. Bacteriophage-typing designations of Salmonella typhimurium. J Hyg (Lond) 1977;78:297–300. doi: 10.1017/s0022172400056187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Palmgren H, McCafferty D, Aspan A et al. Salmonella in sub-Antarctica: low heterogeneity in Salmonella serotypes in South Georgian seals and birds. Epidemiol Infect. 2000;125:257–262. doi: 10.1017/s0950268899004586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Millemann Y, Gaubert S, Remy D, Colmin C. Evaluation of IS200-PCR and comparison with other molecular markers to trace Salmonella enterica subsp. enterica serotype typhimurium bovine isolates from farm to meat. J Clin Microbiol. 2000;38:2204–2209. doi: 10.1128/jcm.38.6.2204-2209.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Girdwood RW, Fricker CR, Munro D, Shedden CB, Monaghan P. The incidence and significance of Salmonella carriage by gulls (Larus spp.) in Scotland. J Hyg (Lond) 1985;95:229–241. doi: 10.1017/s0022172400062665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bengtsson K, Blomquist L. Ursprung, rörelser och ortstrohet för skrattmåsar (Larus ridibundus) märkta i Malmö [in Swedish with English summary] Ornis Svecica. 2001;11:59–77. [Google Scholar]
- 38.Monaghan P, Shedden CB, Ensor K, Fricker CR, Girdwood RWA. Salmonella carriage by Herring gulls in the Clyde area of Scotland in relation to their feeding ecology. J Appl Ecol. 1985;22:669–680. [Google Scholar]
- 39.Bengtsson K. Flyttvägar och övervintringsplatser för svenska skrattmåspopulationer [in Swedish with English summary] Ornis Svecica. 1996;6:17–38. [Google Scholar]
- 40.Tauni MA, Österlund A. Outbreak of Salmonella typhimurium in cats and humans associated with infection in wild birds. J Small Anim Pract. 2000;41:339–341. doi: 10.1111/j.1748-5827.2000.tb03214.x. [DOI] [PubMed] [Google Scholar]
- 41.Hurvell B, Borg K, Gunnarsson A, Jevring J. Studies on Salmonella typhimurium infections in passerine birds in Sweden. Int Congr Game Biol. 1974;11:493–497. [Google Scholar]
- 42.Nielsen B, Clausen B. The incidence of Salmonella bacteria in Danish wildlife and in imported animals. Nord Vet Med. 1975;27:633–640. [PubMed] [Google Scholar]
- 43.Kirkwood JK, Holmes JP, Macgregor S. Garden bird mortalities. Vet Rec. 1995;136:372. doi: 10.1136/vr.136.14.372. [DOI] [PubMed] [Google Scholar]
- 44.Mikaelian I, Daignault D, Duval MC, Martineau D. Salmonella infection in wild birds from Quebec. Can Vet J. 1997;38:385. [PMC free article] [PubMed] [Google Scholar]
- 45.Hudson CR, Quist C, Lee MD et al. Genetic relatedness of Salmonella isolates from domestic birds in southeastern United States. J Clin Microbiol. 2000;38:1860–1865. doi: 10.1128/jcm.38.5.1860-1865.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pennycott TW, Ross HM, McLaren LM, Park A, Hopkins GF, Foster G. Causes of deaths of wild birds of the family Fringillidae in Britain. Vet Rec. 1998;143:155–158. doi: 10.1136/vr.143.6.155. [DOI] [PubMed] [Google Scholar]
- 47.Cramp S, Simmons KEL. The birds of the western Palearctic. III. Oxford: Oxford University Press; 1990. , vol. [Google Scholar]
- 48.Bender JB, Hedberg CW, Boxrud DJ et al. Use of molecular subtyping in surveillance for Salmonella enterica serotype typhimurium. N Engl J Med. 2001;344:189–195. doi: 10.1056/NEJM200101183440305. [DOI] [PubMed] [Google Scholar]
- 49.Jeoffreys NJ, James GS, Chiew R, Gilbert GL. Practical evaluation of molecular subtyping and phage typing in outbreaks of infection due to Salmonella enterica serotype typhimurium. Pathology. 2001;33:66–72. [PubMed] [Google Scholar]