SUMMARY
To determine factors associated with fetal growth, preterm delivery and stillbirth in an area of high malaria transmission in Southern Malawi, a cross-sectional study of pregnant women attending and delivering at two study hospitals was undertaken. A total of 243 (17·3%) babies were preterm and 54 (3·7%) stillborn. Intra-uterine growth retardation (IUGR) occurred in 285 (20·3%), of whom 109 (38·2%) were low birthweight and 26 (9·1%) preterm. Factors associated with IUGR were maternal short stature [adjusted odds ratio (AOR) 1·6, 95% confidence interval (CI) 1·0–2·5]; primigravidae (AOR 1·9, 95% CI 1·4–2·7); placental or peripheral malaria at delivery (AOR 1·4, 95% CI 1·0–1·9) and maternal anaemia at recruitment (Hb <8 g/dl) (AOR 1·9, 95% CI 1·3–2·7). Increasing parasite density in the placenta was associated with both IUGR (P=0·008) and prematurity (P=0·02). Factors associated with disproportionate fetal growth were maternal malnutrition [mid-upper arm circumference (MUAC) <23 cm, AOR 1·9, 95% CI 1·0–3·7] and primigravidae (AOR 1·8, 95% CI 1·0–3·1). Preterm delivery and stillbirth were associated with <5 antenatal care visits (AOR 2·2, 95% CI 1·3–3·7 and AOR 3·1, 95% CI 1·4–7·0 respectively) and stillbirth with a positive Venereal Disease Research Laboratory (VDRL) test (AOR 4·7, 95% CI 1·5–14·8). Interventions to reduce poor pregnancy outcomes must reduce the burden of malaria in pregnancy, improve antenatal care and maternal malnutrition.
INTRODUCTION
Intra-uterine growth retardation (IUGR) and preterm have been implicated in increasing neonatal and infant morbidity [1, 2] and mortality [3, 4] in developed and developing countries. They are also associated with poor neurological and cognitive functioning [5–7] and can contribute to poor growth [8]. Although the importance of IUGR is recognized, there is little information available from developing countries on the frequency of the different types of growth retardation (i.e. proportionate or disproportionate), their risk factors, or how these relate to other poor pregnancy outcomes such as preterm delivery and stillbirth.
The aim of this study was to investigate factors associated with these outcomes for deliveries occurring in a rural malarious area of southern Malawi. The analysis extends previous work on factors related to fetal growth retardation in this population [9].
METHODS
Study area
This study was undertaken between March 1993 and July 1994 in Chikwawa District, in the Shire Valley, Malawi. This is a rural area with high malaria transmission. The infant mortality rate in this district was 174 compared to a national average of 159 deaths per 1000 live births [10]. Small-scale agriculture of maize, sorghum, cotton and sugar cane are the primary source of food and income. The study was located in the two district hospitals in Chikwawa (CDH), a government hospital with free services and Montfort (MH), 30 km away, which is a fee-paying mission hospital.
Study procedures
All women attending the antenatal facilities at CDH or MH between March 1993 and June 1994 were screened at their first antenatal visit after verbal consent was obtained, and subsequently at delivery if this occurred in hospital. A questionnaire, completed by a project nurse included information on age, obstetric history and antimalarial use in pregnancy. Information from obstetric records was collated. Height (cm) in bare feet with no head cover, was measured using a Minimeter [11] and maternal weight (kg) using a SECA scale, and birthweight (g) using a Salter scale. Right mid-upper arm circumference (MUAC) was measured (mm) with a tape measure. A peripheral maternal blood sample was collected at recruitment and delivery for laboratory investigation and also from the placenta. Gestational age was assessed by trained nurses using a modified Ballard scale which had been validated against the full Dubowitz gestational age assessment [12]. All women were advised to deliver in the hospital.
Laboratory investigations
Haemoglobin (Hb) was measured in duplicate by the cyanomethaemoglobin method, packed cell volume (PCV) by microhaematocrit and erythrocyte protoporphyrin (EP) in μg ZP/g Hb, by haematofluorometry (AVIV). Mean corpuscular haemoglobin concentration (MCHC) was calculated. Malaria slides were stained with Giemsa and read counting asexual Plasmodium falciparum parasites against 200 white blood cells. Maternal HIV status was determined using two enzyme-linked immunosorbent assays (ICE*HIV-1.0.2, Murex; Dartford, UK, and for confirmation, VIDAS HIV-2 new test, bioMérieux; Lyon, France). Pre- and post-test counselling was provided by trained nurses. HIV status was measured in a random selection of 600 delivery sera which represented 40% of deliveries.
Definitions
Anaemia was defined as Hb <8 g/dl, low birthweight (LBW) <2500 g, prematurity <37 weeks gestation and IUGR as <10th percentile of the birthweight-for-gestational age sex-specific curve [13]. Disproportionate growth was defined as a ponderal index (weight/length3) <10% of the reference value, and proportionate growth as equal to or above that value [14]. Adolescents were <20 years of age. A MUAC of <23 cm categorized poor maternal nutritional status [15]. Body mass index (BMI) was calculated as kg/m2 from measurements of women who attended initially in the first trimester. Iron deficiency anaemia was defined as an EP of >3·1 μg ZP/g Hb and a MCHC of <32 g/dl [16]. Cord Hb <12·5 g/dl was considered fetal anaemia [17]. Stillbirths were ascertained from the obstetric history or, for late stillbirths, at the time of delivery.
Sample size
To observe a two-fold increased risk of IUGR or preterm delivery associated with placental or delivery malaria, with 95% confidence and 80% power, 225 women with and without placental or delivery malaria were required. We assumed a 30% prevalence of placental or delivery malaria among mothers of babies with IUGR and preterm delivery.
Analysis
Data were analysed using SPSS for Windows, version 11.0 (SPSS Inc., Chicago, IL, USA). The χ2 and Fisher’s exact tests were used for comparison of discrete variables and the Mantel–Haenszel χ2 test for measuring linear association. Analysis of variance was used to compare continuous variables. Logistic regression was used to analyse factors associated with the main outcome variables. Variables included in the model were factors in the univariate analysis which were significant at a P value of <0·05. Malaria parasitaemia was included in all the models. Interaction was assessed between each factor and all the variables in the model. Twins and stillbirths were excluded from the analysis for IUGR, disproportionate growth and preterm delivery. The analysis to examine risk factors for stillbirths also excluded twins.
Ethical approval
The study was granted ethical approval from the Malawi College of Medicine Research and Ethics Committee in Malawi.
RESULTS
There were 1571 hospital births to 4104 pregnant mothers recruited at antenatal clinics. The gestational age distribution is shown in Table 1. A quarter of mothers screened (25·7%) were HIV positive, 18·5% were adolescents and 27·4% had placental or peripheral malaria at delivery. Prevalence of LBW was 14·9% and IUGR 20·3%. Disproportionate IUGR occurred in 58 babies (4·2%), and preterm birth in 243 (17·3%) (Table 2). Prevalence for all outcomes was highest in first pregnancies and significantly decreased with increased parity. There were 54 (3·7%) stillbirths and 43 (5·7%) twin deliveries. Maternal HIV status was not associated with any of the analysed pregnancy outcome variables. Women who delivered in hospital were significantly more likely to be thinner (P=0·02), literate (P<0·001) and primigravidae (P=0·005), although they were less likely to have used intermittent preventive antimalarial treatment during their pregnancy (P<0·001).
Table 1.
Gestational age at delivery
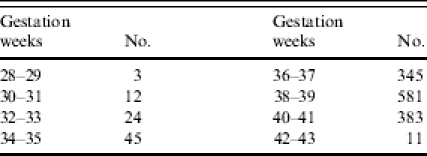
Table 2.
Parity and prevalence of pregnancy outcomes

IUGR, Intra-uterine growth retardation; LBW, low birthweight.
IUGR
Mothers delivering babies with IUGR had lower mean age, BMI, MUAC, weight at recruitment, height, gestation, and Hb at recruitment and delivery (all P<0·05) (Table 3). Primigravidae and adolescents were more likely to have growth-retarded babies than multigravidae (OR 2·3, 95% CI 1·7–2·00 and OR 2·1, 95% CI 1·5–2·8 respectively). Placental malaria (OR 1·6, 95% CI 1·1–2·2), peripheral malaria (OR 1·6, 95% CI 1·2–2·1) or placental and peripheral malaria (OR 1·6, 95% CI 1·2–2·2) were all associated with IUGR. There was an increasing IUGR incidence with higher placental parasite densities (P=0·008) and with increasing parasitaemia in the peripheral blood at delivery (P=0·005) (Table 4). Maternal anaemia (Hb <8 g/dl) at delivery (OR 1·5, 95% CI 1·0–2·2), or at recruitment (OR 1·8, 95% CI 1·3–2·4), were associated with IUGR. Factors independently associated with IUGR were short stature, first pregnancy, placental or peripheral malaria and maternal anaemia (Table 5).
Table 3.
Maternal and child characteristics in relation to intra-uterine growth retardation (IUGR), disproportionate growth and preterm deliveries

(s.d.), Standard deviation; [n], sample size; MUAC, mid-upper arm circumference; ANC, antenatal care; MCHC, mean corpuscular haemoglobin concentration; EP, erythrocyte protoporphyrin.
P<0·05; * P<0·01 for difference between comparison groups.
Table 4.
Malaria parasite density and incidence of intra-uterine growth retardation (IUGR), disproportionate growth, prematurity and stillbirth
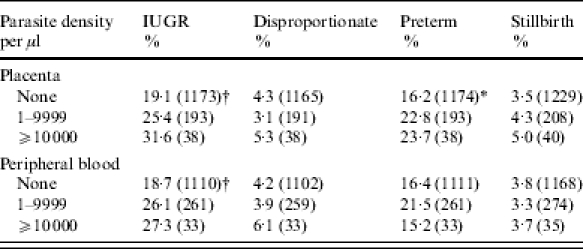
P value for trend: * <0·05; † <0·01.
Table 5.
Logistic regression analysis of factors associated with intra-uterine growth retardation (IUGR), disproportionate growth, preterm birth and stillbirth
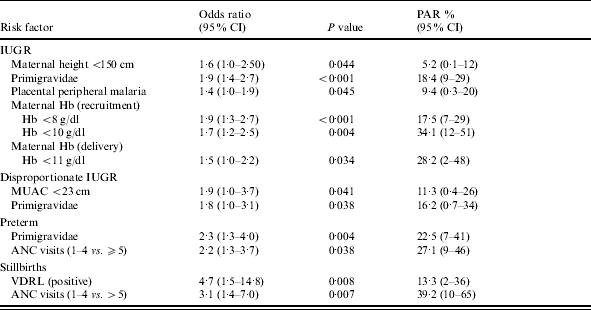
PAR, Population attributable risk; MUAC, mid-upper arm circumference; ANC, antenatal care; VDRL, Venereal Disease Research Laboratory.
Disproportionate IUGR
Mothers with babies with disproportionate growth were younger (P=0·03), had lower MUAC (P=0·04) and lower gestational age (P=0·02) (Table 3). Babies of primigravidae and adolescents were most likely to have disproportionate growth (OR 2·0, 95% CI 1·2–3·4, and OR 1·9, 95% CI 1·1–3·4 respectively), as were those of mothers with poor nutritional status (MUAC <23 cm) (OR 2·1, 95% CI 1·2–4·0). Prematurity occurred more frequently with disproportionate growth (OR 1·9, 95% CI 1·1–3·4). Malaria parasite density showed no association with disproportionate growth (Table 4). In multivariate regression only MUAC <23 cm and first pregnancy remained significantly associated with disproportionate growth (Table 5).
Preterm birth
Factors associated with preterm birth were: adolescence (OR 1·9, 95% CI 1·3–2·6); <5 antenatal visits (OR 2·3, 95% CI 1·7–3·1); short stature (height <150 cm) (OR 1·6, 95% CI 1·1–2·5); MUAC <23 cm (OR 2·0, 95% CI 1·4–2·9); Hb <10 g/dl at recruitment (OR 1·5, 95% CI 1·1–2·2), or <9 g/dl at delivery (OR 1·4, 95% CI 1·0–1·9) and peripheral (OR 1·6, 95% CI 1·1–2·2), or placental and peripheral malaria (OR 1·5, 95% CI 1·1–2·0). For mothers who delivered at CDH, taking ferrous sulphate supplements <5 times (OR 1·9, 95% CI 1·2–2·8) and taking sulphadoxine–pyrimethamine once, instead of twice (OR 1·6, 95% CI 1·0–2·4) were both associated with preterm birth. There was an increasing prevalence of preterm deliveries with increasing placental but not peripheral parasite density (P=0·02) (Table 4). Preterm birth was independently associated with primiparity and <5 antenatal visits (Table 5).
Stillbirths
Mothers with stillbirths had lower MUAC (P=0·008), and lower gestational age (P=0·001) and regression analysis indicated they were more likely to make fewer antenatal care visits (P=0·001) and be positive for the Venereal Disease Research Laboratory (VDRL) test (Table 5). There was no relation between increasing malaria parasite density and occurrence of stillbirths.
DISCUSSION
As women who delivered in hospital were more undernourished than women who did not attend for delivery, the pregnancy outcome may not be fully representative of the whole population. The prevalence of LBW (15%) was high. In Malawi, prevalence of LBW has been reported as 16·8% [18], and in a highly malarious area of southern Tanzania as 18% [19]. The 20·3% prevalence of IUGR in the present study was similar to values from a hospital-based study in Zambia (25·9%). Preterm births occurred with similar frequency to other malarious areas in Africa, for example 17·0% in Zambia [20] and 19·7% in a different region of Malawi [21]. This high incidence clearly indicates a major public health problem with maternal malaria and undernutrition [22].
Maternal HIV infection was not associated with IUGR, preterm birth, or stillbirth. In Ifkara, Tanzania, Menendez et al. also found no association between HIV infection and preterm birth [19], and in Kigali, Rwanda, Leroy et al. reported no independent association between maternal HIV infection, IUGR, or preterm birth, although in univariate analysis there was a two-fold increased risk of both prematurity and IUGR [23]. Taha et al. observed a significantly increased incidence of IUGR and preterm birth in HIV-seropositive mothers in urban Malawi [24], and in Rwanda, an association between maternal HIV infection and IUGR, but not preterm birth, was observed which correlated with birth length, head and chest circumference [25]. A previous meta-analysis reported a two-fold increased risk of IUGR (OR 1·7, 95% CI 1·4–2·0), and preterm birth (OR 1·8, 95% CI 1·6–2·1), and a four-fold increased risk of stillbirth (OR 3·9, 95% CI 2·7–5·8) with maternal HIV infection [26]. In univariate analyses, we have previously reported the odds ratios related to maternal HIV and IUGR (OR 1·2, 95% CI 0·9–1·6), or preterm birth (OR 1·3, 95% CI 0·9–1·8) for this cohort of mothers [9]. The lack of an association in the present study may relate to the small sample size of women tested for HIV, or confounding as maternal HIV infection has been significantly associated with increased malaria prevalence during pregnancy [27, 28].
Maternal short stature and low MUAC were associated with IUGR. Adequate maternal nutritional status, including appropriate weight gain in pregnancy especially in undernourished mothers, is related to improved birthweight outcomes [29, 30]. Approximately 10% of the women had a height <150 cm, and 14% had a MUAC <23 cm, indicating substantial pre-pregnancy maternal malnutrition. Maternal anaemia as factor in IUGR also relates to undernutrition in this area.
Malaria at delivery was independently associated with IUGR in the regression analysis which is consistent with the finding of a higher risk of IUGR with increasing malaria parasite density at delivery. In The Gambia, placental malaria was also significantly associated with IUGR [31], and in Mangochi, southern Malawi, two studies have shown a significant association between placental malaria and IUGR [21, 32]. The exact mechanism leading to IUGR with placental malaria is unclear although placental insufficiency and reduction of oxygen and/or glucose transport to the fetus are likely to play a role. This may relate to a number of mechanisms, for example, mechanical blockage from the thickening of the trophoblast basement membrane, increased nutrient requirements by replicating parasites, or poor oxygen and glucose transfer [33, 34]. Placental abnormalities resulting from nutritional deficiency or maternal vascular changes affecting the placenta may be involved [35]. Pre-eclampsia, which causes reduced placental perfusion could act in a synergistic manner with placental malaria in predisposing to fetal growth restriction [36].
Other factors associated with IUGR in the present study were first pregnancy and maternal anaemia at recruitment or delivery. Primigravidae are more susceptible to falciparum malaria and consequently become anaemic [37, 38]. Primigravidae were three times more likely to have placental or peripheral malaria at delivery and the observed association of maternal anaemia with IUGR is confounded by maternal malaria. The population attributable risk (PAR) of IUGR was 34% for recruitment anaemia and 28% for delivery anaemia. These high PAR values reflect the magnitude of the contribution of malaria and anaemia to IUGR. The low proportion of growth-restricted babies with disproportionate IUGR (4·2%) suggests that fetal growth restriction is chronic, rather than a late pregnancy effect. Factors significantly associated with disproportionate growth were: adolescent primigravidae and poor nutritional status, both of which could lead to late pregnancy fetal growth restriction. Conversely, malaria parasite density was associated with IUGR, but not disproportionate growth, which indicates the importance of chronic placental infections on fetal growth in this population.
No independent association of malaria with preterm delivery was observed, although increasing placental parasite density was associated with prematurity in the univariate analysis. This finding was unexpected as other studies from Africa have reported this finding [19]. The PAR of preterm birth associated with primiparity was nearly 22·5% (Table 4) which is sufficiently high to speculate that there is an additional factor to malaria related to primiparity and which predisposes to preterm birth. This may be co-infections, as a high proportion of these young women are HIV infected [39].
Reduced antenatal care attendance was associated with preterm birth. This partly relates to reduced coverage with the antimalarial sulphadoxine primethamine as intermittent preventive treatment, and with haematinic supplementation for anaemia prevention. In The Gambia, severely anaemic women at 32 weeks gestation were more likely to deliver preterm babies (11·8% vs. 5%), although the difference did not reach significance [40]. The lack of treatment of sexually transmitted infection during pregnancy, with fewer antenatal visits could also be a factor. In South Africa, for example, gonorrhoea has been associated with preterm delivery. Syphilis treatment may be omitted and significantly increases the risk of both preterm birth and stillbirth [41, 42]. However, there is a problem of cause-and-effect in analysing these variables as mothers who had preterm deliveries will also have a shorter time in which to accumulate antenatal visits.
IUGR, preterm delivery and stillbirth commonly occur in this area. Risk factors associated with these pregnancy outcomes are malaria at delivery, maternal anaemia and undernutrition, sexually transmitted diseases, poor antenatal attendance and early age at first pregnancy. Interventions to reduce poor pregnancy outcomes must reduce the burden of malaria in pregnancy, especially in young women, and improve antenatal care and the management of sexually transmitted infections.
ACKNOWLEDGEMENTS
We are grateful for support received from staff at Chikwawa and Montfort hospitals and to B. Makwiza, S. Mlanga, V. Nakoma, M. Gwaza and H. Banda and to laboratory technicians for technical support. Financial support was provided by the Gates Malaria Partnership (GMP) and the European Commission Programme for Life Sciences and Technologies for Developing Countries (contract no. TS3* CT920083).
DECLARATION OF INTEREST
None.
REFERENCES
- 1.Barros FC, Huttley SRA, Victoria CG, Kirkwood BR, Vaughan JP. Comparison of the causes and consequences of prematurity and intrauterine growth retardation: a longitudinal study in southern Brazil. Pediatrics. 1992;90:238–244. [PubMed] [Google Scholar]
- 2.Kabede A, Larson C. The health consequences of intrauterine growth retardation in Southwestern Ethiopia. Trop Doctor. 1994;24:64–69. doi: 10.1177/004947559402400207. [DOI] [PubMed] [Google Scholar]
- 3.Starfield B, Shapiro S, McCormick M, Bross D. Mortality and morbidity in infants with intrauterine growth retardation. J Pediatr. 1982;101:978–983. doi: 10.1016/s0022-3476(82)80025-5. [DOI] [PubMed] [Google Scholar]
- 4.Bernstein IM, Horbar JD, Badger GJ, Ohlsson A, Golan AG. Morbidity and mortality among very-low-birth-weight neonates with intrauterine growth restriction. Am J Obstet Gynecol. 1999;182:198–206. doi: 10.1016/s0002-9378(00)70513-8. [DOI] [PubMed] [Google Scholar]
- 5.Vohr BR, Garcia Coll CT. Neurodevelopmental and school performance of very low-birth-weight infants: a seven year longitudinal study. Pediatrics. 1985;76:345–350. [PubMed] [Google Scholar]
- 6.Lucas A, Morley R, Cole TJ. Randomised trial of early diet in preterm babies and later intelligence quotient. BMJ. 1998;317:1481–1487. doi: 10.1136/bmj.317.7171.1481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hollo O, Rautava P, Korhonen T, Helenius H, Kero P, Sillanpaa M. Academic achievement of small-for-gestational-age children at age 10 years. Arch Pediatr Adolesc Med. 2002;156:171–187. doi: 10.1001/archpedi.156.2.179. [DOI] [PubMed] [Google Scholar]
- 8.Arifeen SE, Black RE, Caufield LE et al. Infant growth patterns in the slums of Dhaka in relation to birth weight, intrauterine growth retardation and prematurity. Am J Clin Nutr. 2000;72:1010–1017. doi: 10.1093/ajcn/72.4.1010. [DOI] [PubMed] [Google Scholar]
- 9.Verhoeff FHL, Brabin BJ, van Buuren S et al. An analysis of intrauterine growth retardation in rural Malawi. Eur J Clin Nutr. 2001;55:682–689. doi: 10.1038/sj.ejcn.1601200. [DOI] [PubMed] [Google Scholar]
- 10.National Statistical Office. 1998. Malawi Population and Housing Census. Summary of Final Results. NSO, Zomba, Malawi.
- 11.Cox LA. A guide to measurement and assessment of growth in children. Welwyn Garden City: Castlemead Publications; 1992. [Google Scholar]
- 12.Verhoeff FH, Milligan P, Brabin BJ, Mlanga S, Nakoma V. Gestational age assessment by nurses in a developing country using the Ballard method, external criteria only. Ann Trop Paediatr. 1997;17:333–342. doi: 10.1080/02724936.1997.11747907. [DOI] [PubMed] [Google Scholar]
- 13.Williams RL, Creasy RK, Cunningham GC, Hawes WE, Norris FD, Tashiro M. Fetal growth and perinatal viability in California. Obstet Gynecol. 1982;59:624–632. [PubMed] [Google Scholar]
- 14.Lubchenco LO, Hansman C, Boyd E. Intrauterine growth in length and head circumference as estimated from live births at gestational ages from 26 to 42 weeks. Pediatrics. 1966;37:403–408. [PubMed] [Google Scholar]
- 15.World Health Organisation. Working Group on Infant Growth. An evaluation of infant growth: the use and interpretation of anthropometry in infants. Bull World Health Organ. 1995;73:165–174. [PMC free article] [PubMed] [Google Scholar]
- 16.Labbe RF, Vreman HJ, Stevenson DK. Zinc protoporphyrin: a metabolite with a mission. Clin Chem. 1999;45:2060–2072. [PubMed] [Google Scholar]
- 17.Brabin BJ. Fetal anaemia in malarious areas: its causes and significance. Ann Trop Paediatr. 1992;12:303–310. doi: 10.1080/02724936.1992.11747589. [DOI] [PubMed] [Google Scholar]
- 18.Steketee RW, Wirima JJ, Hightower AW, Slutsker L, Heymann DL, Breman JG. The effect of malaria and malaria prevention in pregnancy on offspring birthweight, prematurity and intrauterine growth retardation in rural Malawi. Am J Trop Med Hyg. 1996;55:33–41. doi: 10.4269/ajtmh.1996.55.33. [DOI] [PubMed] [Google Scholar]
- 19.Menendez C, Ordi J, Ismail MR et al. The impact of placental malaria on gestational age and birthweight. J Infect Dis. 2000;181:1740–1745. doi: 10.1086/315449. [DOI] [PubMed] [Google Scholar]
- 20.Bhat GJ, Mukelebai K, Shastri GN, Tamia C. Anthropometric parameters of Zambian infants at birth. J Pediatr. 1989;35:100–104. doi: 10.1093/tropej/35.3.100-a. [DOI] [PubMed] [Google Scholar]
- 21.Sullivan AD, Nyirenda T, Cullinan T et al. Malaria infection during pregnancy: intrauterine growth retardation and preterm delivery in Malawi. J Infect Dis. 1999;179:1580–1583. doi: 10.1086/314752. [DOI] [PubMed] [Google Scholar]
- 22.de Onis M, Blossner M, Villar J. Levels and patterns of intrauterine growth retardation in developing countries. Eur J Clin Nutr. 1998;52:S5–S15. [PubMed] [Google Scholar]
- 23.Leroy V, Lander J, Nyiraziraje M et al. Effect of HIV-1 infection on pregnancy outcome in women in Kigali, Rwanda, 1992–1994. AIDS. 1998;12:643–650. doi: 10.1097/00002030-199806000-00014. [DOI] [PubMed] [Google Scholar]
- 24.Taha TET, Dallabetta GA, Canner JK et al. The effect of human immunodeficiency virus infection on birthweight and infant and child mortality in urban Malawi. Int J Epidemiol. 1995;24:1022–1029. doi: 10.1093/ije/24.5.1022. [DOI] [PubMed] [Google Scholar]
- 25.Bulterys M, Chao A, Munyemana S et al. Maternal human immunodeficiency virus 1 infection and intrauterine growth retardation: a prospective cohort study in Butare, Rwanda. Pediatr Infect Dis. 1994;13:94–100. doi: 10.1097/00006454-199402000-00003. [DOI] [PubMed] [Google Scholar]
- 26.Brocklehurst P, French R. The association between maternal HIV infection and perinatal outcome: a systematic review of the literature and meta-analysis. Br J Obstet Gynecol. 1998;105:836–848. doi: 10.1111/j.1471-0528.1998.tb10227.x. [DOI] [PubMed] [Google Scholar]
- 27.Verhoeff FH, Brabin BJ, Hart CA, Chimsuku L, Kazembe P, Broadhead R. Increased prevalence of malaria in HIV infected pregnant women and its implications for malaria control. Trop Med Int Health. 1999;4:5–12. doi: 10.1046/j.1365-3156.1999.00349.x. [DOI] [PubMed] [Google Scholar]
- 28.Steketee RW, Nahlen BL, Parise ME, Menendez C. The burden of malaria in pregnancy in malaria endemic areas. Am J Trop Med Hyg. 2001;64:28–35. doi: 10.4269/ajtmh.2001.64.28. [DOI] [PubMed] [Google Scholar]
- 29.Kramer MS. Determinants of low birthweight: methodological assessment and meta-analysis. Bull World Health Organ. 1987;65:663–737. [PMC free article] [PubMed] [Google Scholar]
- 30.Schieve LA, Cogswell ME, Scanlon KS et al. Pre-pregnancy body mass index and pregnancy weight gain: associations with preterm delivery. Obstet Gynecol. 2000;96:194–200. doi: 10.1016/s0029-7844(00)00883-8. [DOI] [PubMed] [Google Scholar]
- 31.Okoko BJ, Ota MO, Yamuah LK et al. Influence of placental malaria infection on foetal outcome in the Gambia. Twenty years after Ian McGregor. J Health Popul Nutr. 2002;20:4–11. [PubMed] [Google Scholar]
- 32.Moorman AM, Sullivan AD, Rochford RA et al. Malaria and pregnancy: placental cytokine expression and its relationship to intrauterine growth retardation. J Infect Dis. 1999;180:1987–1993. doi: 10.1086/315135. [DOI] [PubMed] [Google Scholar]
- 33.Brabin BJ, Romagosa C, Abdelgalil S et al. The sick placenta – the role of malaria. Placenta. 2004;25:359–378. doi: 10.1016/j.placenta.2003.10.019. [DOI] [PubMed] [Google Scholar]
- 34.Brabin BJ, Kalanda BF, Verhoeff FH, Chimsuku LH, Broadhead RL. Risk factors for fetal anaemia in a malarious area of Malawi. Ann Trop Paediatr. 2004;24:311–321. doi: 10.1179/027249304225019136. [DOI] [PubMed] [Google Scholar]
- 35.Bernardini I, Evans MI, Nicolaides KH, Economides DL, Gahl WA. The fetal concentrating index as a gestational age-dependent measure of placental dysfunction in intrauterine growth retardation. Am J Obstet Gynecol. 1991;164:1481–1487. doi: 10.1016/0002-9378(91)91427-x. [DOI] [PubMed] [Google Scholar]
- 36.Brabin BJ, Johnson PM. Placental malaria and pre-eclampsia – through the looking glass backwards? J Reprod Immunol. 2005;65:1–15. doi: 10.1016/j.jri.2004.09.006. [DOI] [PubMed] [Google Scholar]
- 37.Brabin BJ. An analysis of malaria in pregnancy in Africa. Bull World Health Organ. 1983;61:1005–1016. [PMC free article] [PubMed] [Google Scholar]
- 38.Shulman CE, Graham WJ, Jilo H et al. Malaria is an important cause of anaemia in primigravidae: evidence from a district hospital in coastal Kenya. Trans R Soc Trop Med Hyg. 1996;90:535–539. doi: 10.1016/s0035-9203(96)90312-0. [DOI] [PubMed] [Google Scholar]
- 39.Brabin L, Verhoeff FH, Kazembe P, Brabin BJ, Chimsku L, Broadhead R. Improving antenatal care for pregnant adolescents in southern Malawi. Acta Obstet Gynecol Scand. 1998;77:402–409. [PubMed] [Google Scholar]
- 40.D’Alessandro U, Langerock P, Bennett S, Francis N, Cham K, Greenwood BM. The impact of a national impregnated bed net programme on the outcome of pregnancy in primigravidae in The Gambia. Trans R Soc Trop Med Hyg. 1996;90:487–492. doi: 10.1016/s0035-9203(96)90289-8. [DOI] [PubMed] [Google Scholar]
- 41.Donders GGG, Desmyter J, De Wet DH, van Assche GA. The association of gonorrhoea and syphilis with premature birth and low birthweight. Genitourin Med. 1993;69:98–101. doi: 10.1136/sti.69.2.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Watson-Jones D, Changalucha J, Gumodoka B et al. Syphilis in pregnancy in Tanzania. Impact of maternal syphilis on outcome of pregnancy. J Infect Dis. 2002;186:940–947. doi: 10.1086/342952. [DOI] [PubMed] [Google Scholar]


