SUMMARY
The chemotactic response of Vibrio cholerae O1 towards the mucilaginous sheath of Anabaena sp. was investigated by capillary tube method using a virulent strain of V. cholerae O1, El Tor, Ogawa (3083-T) and its isogenic mutant (HAP-1-T) that lacks the hap gene, which codes for mucinase (HA/protease). Homogenates of Anabaena sp. and purified mucin were used in this study as chemoattractants. Results showed 5·7% bacterial accumulation of wild-type V. cholerae O1 towards 4% homogenates of Anabaena sp. whereas, its mutant (hap−) showed 2·9% accumulation after 90 min. The higher percentage of attraction of wild-type V. cholerae O1 than the mutant (hap−) towards mucin and the homogenates of Anabaena sp. might be due to the activity of mucinase. These results indicate the role of mucinase in the chemotactic motility of V. cholerae O1 towards Anabaena sp.
INTRODUCTION
A blue-green alga, Anabaena sp. can provide a microenvironment for long-term survival of V. cholerae O1 in both the microcosm and the aquatic environment of Bangladesh [1]. In a recent study, it was demonstrated that mucinase, a soluble haemagglutinin/protease (HA/protease) present in both classical and El Tor biotypes, played an important role in the association of V. cholerae O1 with Anabaena sp. [2].
The mucilaginous sheath of blue-green algae has been considered as a potential microenvironment for bacteria. Paerl and Keller [3] observed 1000- to 5000-fold more motile, rod-shaped, Gram-negative bacteria attached to the heterocyst of both Anabaenaoscillarioides and Aphanizomenon flos-aquae than in water columns of eutrophic lakes. Paerl [4] hypothesized that chemotaxis might be functional in making the association between cyanobacteria and bacteria.
Paerl and Gallucci [5] demonstrated the interaction between Gram-negative bacteria and the filaments of A. oscillarioides. They showed that when bacteria approached the filaments, motility in terms of flagellar movement increased markedly over planktonic conditions. When they encountered the heterocyst–vegetative cell junction, flagellar rotation increased for approximately 5–10 min and upon attachment flagellar movement appeared functionally stopped. At this stage, both the host and the epiphytes started to grow [5].
Heterocyst–vegetative cell junctions often excrete copious amount of mucilage, which contains carbohydrates, peptides [6] and free amino acids [7]. All these compounds of Anabaena culture filtrates were found to be chemotactically attracted by the bacteria when they remained free in the microenvironment. Therefore, in the present study, we attempted to compare the chemotactic response of both wild-type V. cholerae O1 (hap+, i.e. mucinase positive) and its isogenic mutant V. cholerae O1 (hap−, i.e. mucinase negative) to the mucilaginous sheath of Anabaena sp.
MATERIALS AND METHODS
Two strains of V. cholerae O1 were used in this study. A wild, hypertoxigenic strain of V. cholerae O1, El Tor, Ogawa (3083-T) containing the hap gene (HA/protease), was isolated from a cholera patient in Saigon, Vietnam, in 1964 [8]. The other strain (HAP-1-T) was an isogenic mutant of V. cholerae O1, El Tor Ogawa (3083-T), which was HA/protease negative, i.e. the hap gene deleted [9].
Partially purified type III mucin (Sigma Chemical Co., St. Louis, MO, USA), extracted from porcine stomach was used in this study. The wash medium used in this study was phosphate buffered saline (PBS, pH 7·4).
V. cholerae O1 preserved in T1Nl soft agar was used for the preparation of bacterial suspension and the chemotaxis experiments were carried out following the procedure described earlier [10, 11].
Anabaena used in this study was maintained as pure culture in standard algal media. Four different concentrations of Anabaena homogenates were prepared, i.e. 0·5, 1·0, 2·0 and 4·0% following the procedure described earlier [11] and were stored in different Eppendorf tubes for the chemotaxis assay.
Mucin (0·05 g) was carefully weighed and mixed with 1 ml pre-sterilized PBS in an Eppendorf tube by vortex. Thus, 5·0% stock solution of mucin was prepared. From the stock solution, 1, 2 and 4% mucin solutions were prepared with pre-sterilized PBS for the chemotaxis assay.
A series of pre-sterilized capillary tubes (10-μl Pyrex disposable micro-sampling pipette) were used in the experiment. The capillary tubes were plunged into the Eppendorf tubes containing different concentrations of Anabaena homogenates. The capillary tube was inserted into the syringe containing the bacterial suspension in such a way that the open tips of the capillary tubes were submerged in bacterial suspension. The syringes with the capillary tubes were then kept for 15, 30, 45, 60, 75 and 90 min at room temperature (25°C). As a previous study [11] had shown that chemotactic response of V. cholerae and Anabaena homogenate was highest at 25°C, all the experiments in the present study were carried out at room temperature (25°C). The room temperature was controlled by using an air conditioner. These procedures were followed as described by Adler [10] and Rahman et al. [11]. Ten-fold dilutions of the chemotaxis medium containing V. cholerae O1 were prepared and the samples were plated on TTGA medium following the drop-plate technique [12]. After incubation for 18–24 h at 37°C, colonies of V. cholerae O1 were counted. Each experiment was repeated three times and the mean count was recorded. Capillary tubes with control (PBS) were also used in this experiment. The number of bacteria entered into the capillary tube was calculated as per cent accumulation using the formula described earlier [10, 11].
RESULTS
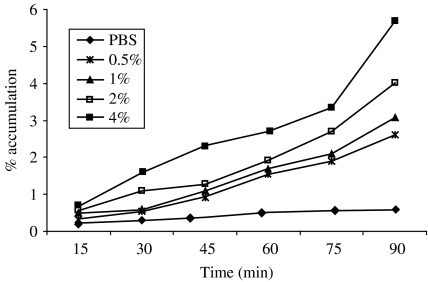
The accumulation of wild-type V. cholerae O1 (hap+) was observed for different concentrations of homogenates of Anabaena used (Fig. 1). The percentage of accumulation of V. cholerae O1 gradually increased with the increased percentage of Anabaena homogenates. The highest (5·7%) percentage of bacterial accumulation was observed at 4% homogenates of Anabaena sp. at 90 min.
Fig. 1.
Chemotactic response of wild-type V. cholerae O1, El Tor Ogawa (3083-T) towards different concentrations of Anabaena homogenates.
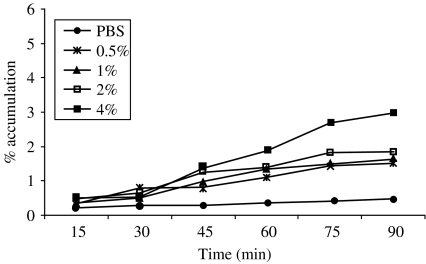
The accumulation of mutant strain was also observed at different concentrations (0·5, 1·0, 2·0 and 4·0%) of homogenates of Anabaena (Fig. 2). The percentage of accumulation was much lower than that observed in wild-type strain (Fig. 1). The highest accumulation (2·9%) was observed for 4% homogenates at 90 min, which is almost 50% lower than the wild-type strain.
Fig. 2.
Chemotactic response of mutant V. cholerae O1, El Tor Ogawa (HAP-1-T) towards different concentrations of Anabaena homogenates.
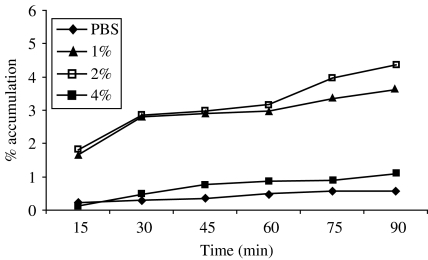
The accumulation of wild-type V. cholerae O1 (hap+) was observed at different concentrations of mucin used (Fig. 3). The highest (4·4%) bacterial accumulation was observed at 2% concentration of mucin after 90 min, whereas in control (PBS), virtually no accumulation was observed, even after 90 min.
Fig. 3.
Chemotactic response of wild-type V. cholerae O1, El Tor Ogawa (3083-T) towards different concentrations of mucin.
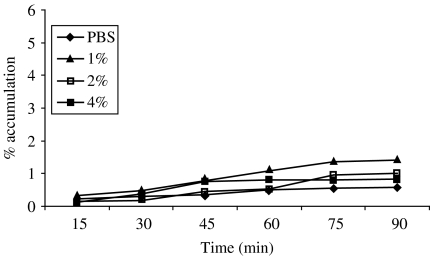
In Figure 4, the percentage accumulation of mutant type V. cholerae O1 (hap−) towards different concentrations of mucin has been depicted. No significant bacterial accumulation was evident towards any concentration of mucin. The highest (1·4%) bacterial accumulation was observed at the lowest (1%) concentration of mucin compared to the higher concentrations of 2 and 4%, which were found at 1 and 0·8% respectively.
Fig. 4.
Chemotactic response of mutant V. cholerae O1, El Tor Ogawa (HAP-1-T) towards different concentrations of mucin.
Discussion
In the present study, results showed that the rates of bacterial accumulation in capillary tubes towards the homogenates of Anabaena sp. were gradually increased with the function of time. The wild-type strain of V. cholerae O1 showed the highest percentage (5·7%) of bacterial accumulation at 4·0% homogenates after 90 min. On the other hand, the mutant strain of V. cholerae O1 (hap−) showed comparatively poor (2·9%) chemotactic response towards 4% homogenates of Anabaena sp. after 90 min. However, no significant bacterial accumulation was observed in the case of control suggesting that wild-type V. cholerae O1 with its ability to produce mucinase showed a stronger chemotactic response than that of the mutant type.
In a recent study [2], it has been demonstrated that the mutant strain of V. cholerae O1 (hap−) could not persist longer in association with Anabaena sp. These findings led to the idea that chemotactic motility of V. cholerae O1 towards the mucilaginous sheath of Anabaena sp. could be influenced or stimulated by the chemoattractant mucin that covered the mucilaginous sheath.
Partially purified mucin extracted from porcine stomach was also used in different concentrations (1, 2 and 4%) for chemotaxis assay. The bacterial accumulation of wild-type strain of V. cholerae O1 increased with exposure to 1 and 2% mucin but was dramatically reduced when exposed to 4% mucin. The change in the direction of flagellar rotation from counterclockwise to clockwise may influence chemotaxis and motility. Armed with a single polar flagellum, V. cholerae can randomly reorient itself and swim in a new direction as described by Butler and Camilli [13] and, therefore, the 4% concentration of mucin may change the V. cholerae flagellar rotation which might cause less accumulation of V. cholerae. Another explanation may be due to rate-limiting of mucinase when exposed to a higher concentration of mucin.
The present study showed that chemotactic motility of V. cholerae O1 towards the mucilaginous sheath of Anabaena sp. might be the function of mucinase (HA/protease). This study provides some insight into the association of V. cholerae O1 with cyanobacteria.
Acknowledgements
This research was funded by ICDDR,B: Centre for Health and Population Research. ICDDR,B is supported by countries and agencies, which share its concern for the health problems of developing countries.
DECLARATION OF INTEREST
None.
REFERENCES
- 1.Islam MS, Drasar BS, Bradley DJ. Long-term persistence of toxigenic Vibrio cholerae O1 in the mucilaginous sheath of a blue-green alga, Anabaena variabilis. J Trop Med Hyg. 1990;93:133–139. [PubMed] [Google Scholar]
- 2.Islam MS, Goldar MM, Morshed MG, Khan MNH, Islam MR, Sack RB. Involvement of the hap gene (mucinase) in the survival of Vibrio cholerae O1 in association with the blue-green alga, Anabaena sp. Can J Microbiol. 2002;48:793–800. doi: 10.1139/w02-073. [DOI] [PubMed] [Google Scholar]
- 3.Paerl HW, Keller P. Significance of bacterial Anabaena (Cyanophyceae) associations with respect to N2 fixation in freshwater. J Phycol. 1978;14:254–260. [Google Scholar]
- 4.Paerl HW. Role of heterotrophic bacteria in promoting N2 fixation by Anabaena in aquatic habits. Microbiol Ecol. 1978;4:215–231. doi: 10.1007/BF02015078. [DOI] [PubMed] [Google Scholar]
- 5.Paerl HW, Gallucci KK. Role of chemotaxis in establishing a specific nitrogen-fixing cyanobacterial-bacterial association. Science. 1985;227:647–649. doi: 10.1126/science.227.4687.647. [DOI] [PubMed] [Google Scholar]
- 6.Fogg GE. The extracellular products of algae. Ocean Marine Biol Ann Rev. 1966;4:195–212. [Google Scholar]
- 7.Walsby AE. The extracellular products of Anabaenacylindrica Lemm. Isolation of a macromolecular pigment peptide complex and other components. Br Phycol J. 1974;9:371–378. [Google Scholar]
- 8.Finkelstein RA, Sobocinski PZ, Atthasampunna P, Charunmethee P. Pathogenesis of experimental cholera: identification of choleragen (procholeragen A) by disc immunoelectrophoresis and its differentiation from cholera mucinase. Infect Immun. 1966;97:25–33. [PubMed] [Google Scholar]
- 9.Häse CC, Finkelstein RA. Comparison of the Vibrio cholerae hemagglutinin/protease and the Pseudomonas aeruginosa elastase. Infect Immun. 1990;58:4011–4015. doi: 10.1128/iai.58.12.4011-4015.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Adler J. A method for measuring chemotaxis and use of the method to determine optimum conditions for chemotaxis by Escherichia coli. J Gen Microbiol. 1973;74:77–91. doi: 10.1099/00221287-74-1-77. [DOI] [PubMed] [Google Scholar]
- 11.Rahman M, Mizanur RM, Khan SI, Hossain MR, Islam MS. Influence of temperature on the chemotaxis of Vibrio cholerae O139 towards Anabaena sp. J Biol Sci. 2003;3:237–246. [Google Scholar]
- 12.Hoben HJ, Somasegoran P. Comparisons of the pour, spread and drop plate method for enumeration of Rhizobium spp. in inoculants made from presterilized peat. Appl Environ Microbiol. 1982;44:1242–1247. doi: 10.1128/aem.44.5.1246-1247.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Butler SM, Camilli A. Both chemotaxis and net motility greatly influence the infectivity of Vibrio cholerae. Proc Natl Acad Sci USA. 2004;101:5018–5023. doi: 10.1073/pnas.0308052101. [DOI] [PMC free article] [PubMed] [Google Scholar]