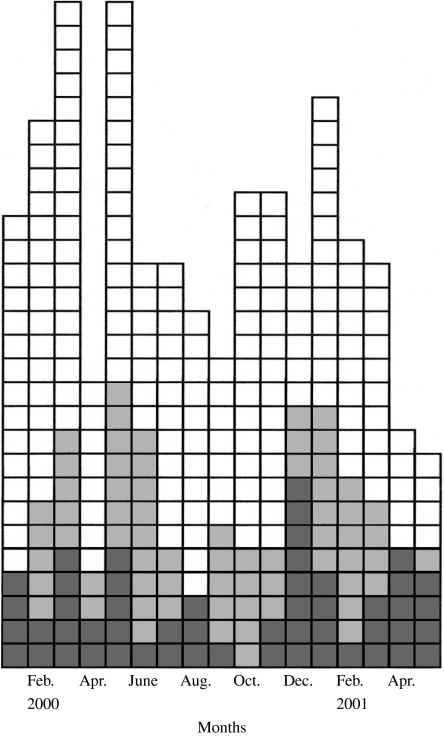SUMMARY
Mass gatherings are believed to increase the transmission of infectious diseases although surveillance systems have shown a low impact. The Catholic Jubilee was held in Rome, Italy in 2000. We conducted a case-control study to analyse the risk factors of giardiasis among residents. All diseases reported to the laboratory surveillance system from January 2000 to May 2001 were compared with hospital controls concurrently selected in the same season as cases and frequency-matched for age and birth country. Fifty-two cases (44·1%) and 72 controls were enrolled. In the multivariable analysis factors associated with giardiasis among adults were: travelling abroad (OR 24·2, P>0·01), exposure to surface water (OR 4·80, P=0·05), high educational level (OR 3·8, P=0·03). Having a maid from a high-prevalence country was independently associated (OR 2·3) although not statistically significant. This is the only exposure that changed during the Jubilee.
INTRODUCTION
Mass gatherings have long been considered a challenge for the transmission of infectious diseases, however, their impact has been reported to be low in developed countries in studies based on surveillance systems [1]. Of the 26 million pilgrims who attended the Catholic Jubilee in Rome in 2000, about 3 million came from developing countries. A slightly higher risk of infectious diseases was detected for pilgrims than for residents [2], based on surveillance system data. To better evaluate the impact of this extraordinary event on residents’ health in Rome, a case-control study was conducted to identify determinants of giardiasis, during and after 2000.
Giardia lamblia, a flagellate protozoan parasite, is a frequent cause of diarrhoea in developing countries as well as in a few industrialized countries [3, 4]. Geographical areas classified as having a high (10–30%) or medium (3–10%) prevalence of giardiasis are Central and South America [5–7], South-East Asia [8, 9], Eastern Europe [10], Africa [11, 12] and the southern Mediterranean [13, 14]. The incubation period of giardiasis lasts 7–10 days, with a range of 5–25 days [4]. The reservoir of Giardia is comprised of humans and both wild and domestic animals. Transmission can occur at any time during the infection period, from either symptomatic or asymptomatic individuals [3]. One study estimated that 25–30% of infected people were asymptomatic [3], and the estimate increases to 76% during epidemics [15]. Transmissibility can last for as long as 6 months [16]. A seasonal trend is reported in temperate areas, with peaks at the end of summer or in autumn [17, 18]. People aged 0–4 and 25–44 years are reported to be at the highest risk [4, 17]. Males are at a slightly higher risk than females [18].
Indirect transmission by drinking water, exposure to surface water or eating contaminated food are the most frequently reported factors associated with outbreaks of giardiasis [19–21], while sporadic cases are most frequently spread by person-to-person transmission, facilitated by close contact such as found in childcare facilities or within households [22, 23]. Waterborne transmission has also been associated with non-outbreak-related cases of giardiasis [22–26].
Among the other diseases with comparable epidemiological characteristics, only salmonellosis and clinically diagnosed infective diarrhoea are surveyed in Italy. These alternatives were both excluded; the former because of the high incidence of salmonellosis in the Lazio region (23 cases/100 000 inhabitants in 1999), the latter because it includes many different diseases since the aetiology of diarrhoea is not detected. All the other diarrhoeic diseases including Shigella and Giardia infections are only reported in Italy during an epidemic. A laboratory-based surveillance for G. lamblia was started in the Lazio region in 1996.
An annual prevalence of 4·1 cases/100 000 inhabitants (213 cases) was estimated in the Lazio region in 1998–1999. No epidemics have been detected here since the reporting of giardiasis outbreaks was made obligatory in 1995.
In conclusion, giardiasis was chosen to study the impact of the Jubilee on residents’ health because of its epidemiological characteristics: a higher incidence in developing countries than in Italy, the possibility of increased transmission from pilgrims to residents during the Jubilee, an incubation period short enough to allow an increased incidence of the disease to be detected, and finally, the availability of an accurate surveillance system.
METHODS
The study was designed as an incident case-control in which all patients who were reported to Giardia laboratory surveillance from January 2000 to May 2001 were enrolled prospectively. Two hospital controls were selected concurrently for each case during the season when cases were diagnosed, and selection continued for 30 days after the end of each season [27].
Ethical approval for the study was obtained from the Ethics Committee of the Catholic University of Rome, who took part in the study.
Case definition
Among the patients positive for G. lamblia (cysts or trophozoites) we included those who were residents of Rome, who were symptomatic within the 60 days before diagnosis, who suffered from diarrhoea (at least three loose stools per day) or had two of the following symptoms: abdominal cramps, foul-smelling and greasy stools, flatulence, bloating, nausea, excessive tiredness, anorexia, and weight loss [28]. A different case definition was adopted for children aged between 2 months and 2 years, based on diarrhoea defined as six or more loose stools per day, and weight loss. The same laboratory confirmed the case diagnosis microscopically. Patients were not included if they had had symptoms for more than 60 days before diagnosis, had been previously diagnosed with a giardiasis, were affected by clinical conditions causing diarrhoea (stomach or intestinal cancer, ulcerative rectocolitis, Crohn’s disease), by immunodeficiency (AIDS, agammaglobulinaemia, other congenital immunodeficiency syndrome, thymoma, asplenia), or diseases for which antiblastic or cortisonic therapy was being administered at dosages of at least 2 mg/day for 14 days.
Control definition
Controls were selected attempting to balance distribution of cases and controls by broad age group, under or over 14 years, and by geographical area of origin, at low or high prevalence of giardiasis. High-prevalence countries included Africa, Asia, South and Central America, and Eastern Europe. Controls were selected from four hospitals in Rome, which were the most important sources of Giardia reports in previous years, among patients hospitalized in orthopaedic, otoryngology and ophthalmology wards. There was one paediatric hospital, two teaching hospitals and one of the three biggest hospitals in Rome.
Controls had to meet the same criteria as cases to be included: they had to be resident in Rome and not present any of clinical conditions listed previously. In addition they had to be hospitalized for fewer than 8 days, not have a previous diagnosis of giardiasis, nor a history of gastrointestinal symptoms in the 60 days before the interview. One to three stool samples were tested for Giardia immediately after the questionnaire was administered. A follow-up of 6 months after enrolment was done for each control through laboratory surveillance to exclude subsequent Giardia infections.
Factors analysed
Information on exposures was gathered for the 4 weeks preceding the date of symptom onset or the interview date for controls, because the incubation period can be as long as 4 weeks for Giardia infection.
The questionnaire included identified risk factors for direct transmission of Giardia such as: attending community or day-care centres [3], practising homosexual intercourse [29], living with bed-ridden people [22] or with diapered children [30], having close contact with domestic or farm animals [31–33]. We added housing or receiving guests during the Jubilee year and having a maid from a high-prevalence country to the direct exposures. Our hypothesis in analysing having a maid from a high-prevalence country did not address whether their numbers had increased during the Jubilee, rather that, during the Jubilee, they were very likely to host pilgrims coming from the same country that they came from, and to work as a vehicle of Giardia infection. Based on this possible scenario, having a maid from a high-prevalence country has been hypothesized as the main exposure for residents during the Jubilee.
The risk factors of indirect contact related to exposure to contaminated environments were: drinking surface water [20, 22] or eating contaminated food [21] assessed on the basis of reporting having picked and eaten raw vegetables, camping, spending time in open spaces or in the country, swimming in pools or natural water [22, 34], travelling to countries with a high prevalence of Giardia infection [35, 36], working in a day-care centre or nursery school or on a sewer system [37]. We used educational level as an indicator of social position.
Sample size
A sample size of 192 cases was estimated, based on a case-control ratio of 1:2, with 95% confidence and 80% power to detect an odds ratio (OR) of at least 3 for the variables under investigation. The underlying hypothesis was that the number of people possibly exposed to effective contact with pilgrims for Giardia infection depended on (i) the number of people expected to visit Rome from high-prevalence countries (nearly 3 million); (ii) the number of residents of Rome coming from high-prevalence countries (nearly 210 000, 4% of residents) expected to house their compatriots; (iii) the number of maids coming from high-prevalence countries (nearly 37 000, 0·7% of residents) who could transmit the infection. Expected cases were estimated according to data from laboratory surveillance during 1998–1999.
Data analysis
Association between giardiasis and each risk factor was estimated through ORs and 95% confidence intervals (CIs). Unconditional logistic regression was carried out, for the adults, to determine the effect of our main exposure controlling for country of origin and risk factors significantly associated with giardiasis according to crude ORs. For model building, we mainly focused on the presence of a maid from a high-prevalence country, forced presence of age, country group and education, and proceeded with backward deletion strategy to select the other risk factors as confounders, using the 0·20 level as the screening criterion. Interactions between the main exposure and all other covariates, including country of origin, were tested. The stata software (version 7, Stata Corporation, TX, USA) was employed for data analysis.
Cases with missing information on exposures were excluded from the analysis.
RESULTS
Three hundred and seven cases were reported in the study period. Among 118 eligible cases, 52 (44%) were enrolled, 21 (19%) refused to participate, and 45 (41%) were not found at the address registered by the laboratory. Enrolled cases differed from non-enrolled by birth country distribution: all non-respondents were from low-prevalence countries. Other differences concerned diagnosis period: there was a higher percentage of non-respondents in spring 2000 (27% compared to 15%) and winter 2001 (27% compared to 17%) (Table 1).
Table 1.
Percentage of respondents among eligible cases by age group, gender, country of birth and enrolment timing
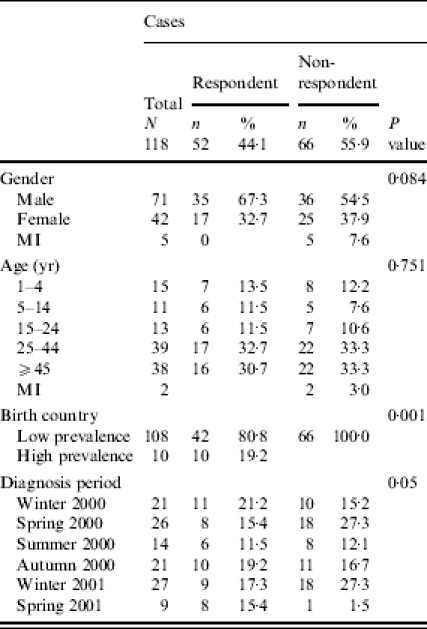
MI, Missing information.
All cases diagnosed in the study period showed an unusual seasonal distribution with peaks in spring and winter (Fig.). All of the cases enrolled were sporadic and had no contact with each other. No person selected as a control later became a case. Cases reported diarrhoea more frequently than other symptoms (64%), followed by abdominal cramps, intestinal gas and excessive tiredness (from 54 to 65%). Among children, 69% reported diarrhoea. The median time between microscopic diagnosis and onset of symptoms was 23·3 days (mean 27·7), and 19 (37%) cases reported symptoms between 30 and 60 days before diagnosis.
Fig.
Epidemic curve of patients positive to microscopic test for Giardia on the basis of diagnosis date, Lazio, January 2000–May 2001. □, 1 case non-eligible;  , 1 case non-respondent;
, 1 case non-respondent;  , 1 case respondent.
, 1 case respondent.
Seventy-two controls were enrolled in the study, only one patient refused to answer the questionnaire. Thirty-nine (54%) had their stools tested microscopically at least once, with negative results for Giardia, both for cysts and trophozoites. Although age groups and prevalence of giardiasis in origin country, which were the frequency-matching criteria, were distributed similarly for respondent cases and controls (Pearson’s χ=4·17, P value=0·24), some differences were observed: 25% of the cases and 15% of the controls were 1–14 years old, and among adults, 26% of cases and 13% of controls were from high-prevalence countries. Cases were enrolled with a median delay of 6 days after diagnosis (mean 16 days). The ratio case-controls was 1:1·7 in all seasons except summer 2000 and spring 2001 in which the ratio rose to 1:0·3 and 1:0·9 respectively.
In the single-variable analysis, five factors were associated with giardiasis among adults from low-prevalence countries: travelling to high-prevalence countries (Central America, Africa, South-East Asia) (OR 25·1, P=0·001), exposure to sewage, manure or animal husbandry (OR 13·3, P=0·02), drinking or bathing in surface water, including swimming pools and natural waters (OR 4·6, P=0·04), spending a day in the country (OR 5·0, P<0·01) and having a maid from a high-prevalence country (OR 5·0, P=0·02). The risk of giardiasis is almost twice as high in people with a high educational level than in those with a low educational level (OR 2·9, P=0·04). The exposure to surface water, even though not statistically significant, was the only factor showing a high risk of giardiasis for adults coming from high-prevalence countries (OR 3·6, P=0·33). Also high educational level showed a somewhat high risk of giardiasis in this group of patients (OR 2·7, P=0·34). No excess risk was found for professional exposure in day-care centres, or having pets, or handling diapered children in adults both from high- and low-prevalence countries (Table 2). Gender distribution was similar among adult cases and controls (P=0·2). Finally, for children 1–14 years old, no factor was significantly associated with an increased risk of giardiasis; however, there were higher risks for keeping pets (OR 4·4, P=0·22), spending a day in the country (OR 3·33, P=0·33), and having a maid from a high-prevalence country (OR 2·2, P=0·54) eating raw vegetables (OR 2·00; P=0·48) and father’s educational level greater than 8 years (OR 2·7, P=0·34) (Table 3).
Table 2.
Crude ORs of giardiasis in adults by prevalence of disease in origin country
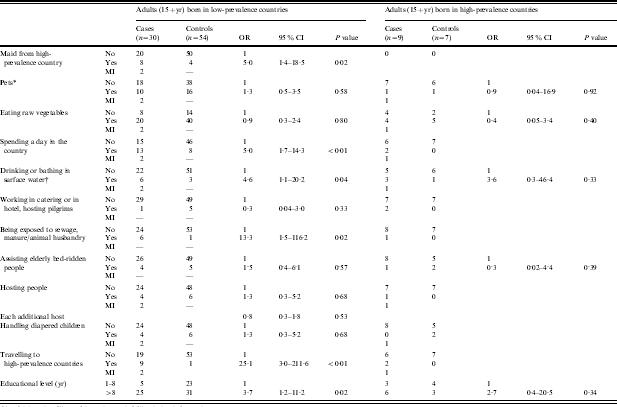
OR, Odds ratio; CI, confidence interval; MI, missing information.
Dogs, cats, guinea pigs.
Including swimming pools and recreational waters.
Table 3.
Crude ORs of giardiasis in children
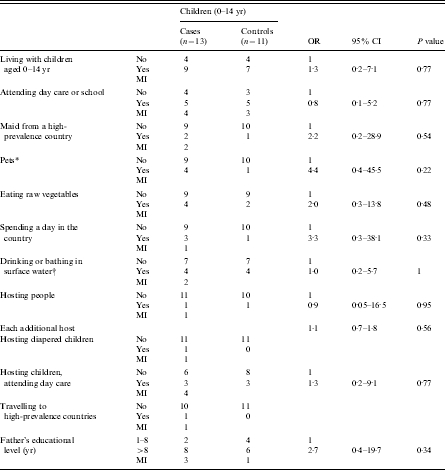
OR, Odds ratio; CI, confidence interval; MI, missing information.
Dogs, cats, guinea pigs.
Including swimming pools and recreational waters.
In the multivariable analysis carried out on adults, higher risk of giardiasis was found for having a maid from a high-prevalence county, although not statistically significant and less high than in single variable analysis; travelling to high-prevalence countries, exposure to surface water, and having a higher educational level were still independently associated with illness (Table 4).
Table 4.
Adjusted ORs of giardiasis in adults (15+ yr)
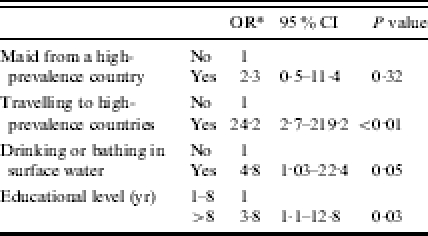
OR, Odds ratio; CI, confidence interval.
Adjusted for age, country and all the other reported variables.
DISCUSSION
Factors associated with giardiasis were travelling to or having a maid from a high-prevalence country, exposure to surface waters and educational level.
Travelling is the most relevant risk factor for giardiasis: it was estimated to be 25 times more frequent in travellers than in those who did not travel to high-prevalence countries. Although this result was expected based on previous studies [34, 36, 38], the estimated risk was very high. We cannot exclude the possibility that the risk could have been overestimated because of a selection bias of respondents among cases, or because a few diseases of controls [congenital deformity of lower limb (n=8), osteoarthrosis (n=5), vertiginous syndromes (n=2)] may have prevented travelling abroad, or because of the difference in exposure time of ∼60 days between enrolment of controls and cases. Nevertheless, a previous study reported an estimated ratio of 22 for travelling to developing countries [34]. Travelling abroad could justify the unusual seasonal distribution of cases, since travel to distant countries is common during Christmas and Easter holidays.
The exposure to surface waters included both drinking and recreational exposure. Drinking improperly treated water was found to be an effective risk for giardiasis in a few studies [23–25, 34], but not in others [20, 38]. Specific environmental characteristics might have influenced the exposure dose and justified the differences in risk estimates. The exposure to swimming pools and natural bodies of water has been more consistently reported in many studies [19, 22, 26, 34, 38]. The risk we observed for surface water is lower than reported in studies from the United States, England or Australia, but it is still appreciable after taking into account the other exposures. Therefore, the observed differences could be due to the smaller prevalence of exposure to surface water in the Lazio region as indicated by the proportion of controls exposed: 6% in Italy compared to 40% in Australia, 31% in England, and 17% in the United States.
The second exposure analysed here implies an indirect transmission of Giardia infection. It could explain better the proportion of endemic giardiasis in our region [22–24, 26]. The association of the disease with exposures working by direct transmission, such as having a maid from a high-prevalence country or hosting guests from such countries could have better estimated the impact of pilgrims on incident giardiasis. The observation of independence of this risk factor from the others working by indirect transmission, travelling abroad or drinking surface water, supports the view that a direct transmission could be suggested for having a maid from a high-prevalence country.
This factor was associated with giardiasis in multivariable analysis, although it was not statistically significant. This was probably due to the small numbers and its high co-linearity with both travelling abroad and, particularly, high educational level. Moreover, we cannot exclude that these results could have been affected by selection bias due to cases not enrolled. In fact, assuming that from the 66 cases who were not enrolled, the proportion of subjects with a maid from a high-prevalence country had been half of those enrolled, the estimated risk ratio would equal 2·96, but if we accepted the very unlikely hypothesis that none of the non-enrolled cases had been exposed there would be no increased risk.
The higher risk of giardiasis in adults with higher education could be related to a situation of endemic infection and low prevalence, as in the Lazio region. Because of oral–faecal transmission, a higher prevalence is expected in children and in lower social classes, but adults with higher educational levels could show a higher incidence when exposed because they are more susceptible. It is more difficult to interpret the higher educational level of cases found among children and people from high-prevalence countries. Therefore, we cannot exclude that a selection bias occurred during case enrolment, since non-respondents were more likely to have lower educational level; while we exclude the possibility of a selection for hospitalized controls with lower education because the proportion of people with <9 years of schooling was 60% among hospitalized people and 62% in the whole population. In other studies, social class had a similar distribution in cases and controls [26, 32] and when higher risk was reported this was linked to exposure to diapered children [38].
At single-variable analysis, two other factors showed a strong association with giardiasis: professional exposure and spending a day in the country.
The association of giardiasis with professional exposure to human waste has already been reported [37, 38] but with risk estimates smaller than those found in this study. The difference could be due to our including exposure to animal waste. An association with these exposures was reported previously [32] but another study failed to observe an increased risk of giardiasis because of contact with farm animals [24]. Until recently the exposure both to farm animals and pets has been little studied because the biological plausibility of transmission from animals to humans was in doubt. The potential risk of infection has been proven by a few studies using molecular analyses on the basis of the morphological similarity of Giardia isolated from humans and other mammals [31, 33].
Spending a day in the country was associated with giardiasis in this study although not independently from other variables. This result is in agreement with the increased risk of giardiasis reported in other studies due to camping or caravanning [22, 34]. Both of these studies suggest that a country environment could be the source of Giardia infection through animal waste. Recent findings show that soil may play a larger role in transmitting enteric diseases than previously thought as it can work as a vector and reservoir of pathogens including protozoa [39].
The difficulty in appreciating the risk for spending time in the country as well as the professional exposure in the multivariable analysis could be due to the power of the study. On the other hand, an insufficient comparability of exposure time between cases and controls could have overestimated the risks. These exposures are both more frequent during spring than other seasons. The enrolment of controls was delayed up to ∼60 days compared to cases because a median of 23·3 days elapsed between diagnosis and onset of symptoms and up to another 30 days could elapse between enrolment of cases and controls. Moreover, a recall bias was possible, as cases knew their diagnosis when the questionnaire was administered.
This study presents a few limits. Non-response bias is a major limitation of the validity of the study. Nevertheless the response rate was low among cases (44·1%), respondents and non-respondents were similar for age and gender. On the other hand, a smaller percentage (81%) of cases were from low-prevalence countries while all those from high-prevalence countries were enrolled; this could have caused a bias and supports the choice of including the country of origin in the model among the confounding variables.
A recall bias as well as inverse causation could have been possible. Since both cases and interviewers knew the diagnosis when the questionnaire was administered, they both may have been more likely to report potentially harmful exposures than the control group. The most common exposures, like salad consumption, could have occurred before or after the infection, during the 30 days before the onset of diarrhoea.
Another important limit is the weak power of the study due to the small number of enrolled cases. Although other case-control studies have been carried out with a small number of cases [25, 32, 34] this study size enabled us to detect more precisely the main risk factor we hypothesized as linked to the Jubilee period, i.e. having a maid from a developing country, who in turn had contact with pilgrims during the Jubilee. To detect this kind of exposure the sample size would have to be 192 or more; the enrolled cases numbered 52.
Finally, controls had to have a negative stool test for Giardia. Only 54% were analysed, all with negative results. On the other hand, no other study tested controls for Giardia [20, 22, 24, 32, 34, 38], the prevalence of infection was as low as 4/100 000 inhabitants, and it was reported that up to five or six stool samples may be examined without recovering the organism in infected people because the parasites mechanically adhere to the intestinal mucosa [40].
We can conclude that the Jubilee had a limited impact on the epidemiology of giardiasis. The increased risk of giardiasis with the presence of a maid from a high-prevalence country, although suggestive of an important exposure did not reach statistical significance, possibly because the sample was too small.
Other risks strongly associated with giardiasis were detected, but they were not linked with the Jubilee. Travelling is the most important risk factor of giardiasis for residents in the Lazio region.
APPENDIX. The Regional Network of Laboratory Surveillance of Giardia group
Carmen Bonanno (Sandro Pertini Hospital), Michela Carletti (Bambino Gesù Hospital), Roberta Cavarischia (Villa Betania Hospital), Alessandra Di Tullio (Nuovo Regina Margherita Hospital), Germana Formosa (San Filippo Neri Hospital), Giulia Gilardi (Forlanini Hospital), Laila Pacciani (San Giacomo Hospital), Paolo Culotta (CID Laboratory), Francesca D’Antonio (Flaminio 9 Laboratory), Mirella Macigno (Centro Ricerca Studio Laboratory), Luca Marino and Luisa Napoli (Marilab Laboratory), Filippo Ranfi (Mercury Laboratory), Giuditta Valorani (Bios Laboratory), Luigia Cortese (Ars Medica Private Hospital), Antonella Di Luzio (Guarnieri Private Hospital), Giulio La Greca (Rome American Hospital, private hospital), Claudio Romagnoli (Madonna della Fiducia, private hospital, Rome, Italy).
ACKNOWLEDGEMENTS
We thank Margaret Becker for the English revision of the manuscript.
DECLARATION OF INTEREST
None.
REFERENCES
- 1.Jorm LR, Thackway SV, Churches TR, Hills MW. Watching the games: public health surveillance for the Sydney 2000 Olympic games. J Epidemiol Commun Health. 2003;57:102–108. doi: 10.1136/jech.57.2.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Giorgi-Rossi P, Sangalli M, Faustini A, Forastiere F, Perucci C. Infectious diseases in Rome during the Millennium year. Eurosurveillance. 2003;8:181–185. doi: 10.2807/esm.08.09.00425-en. [DOI] [PubMed] [Google Scholar]
- 3.Hill DR, Mandell GL, Bennett JE, Dolin R. Mandell, Douglas, Bennett’s principles and practice of infectious diseases. 5th edn. Edinburgh: Churchill Livingston; 2000. Giardia lamblia; pp. 2888–2892. [Google Scholar]
- 4.Chin J. Control of communicable diseases manual. 17th edn. Washington, DC: APHA; 2000. pp. 220–222. [Google Scholar]
- 5.Tellez A, Morales W, Rivera T, Meyer E, Leiva B, Linder E. Prevalence of intestinal parasites in the human population of Leon, Nicaragua. Acta Trop. 1997;66:119–125. doi: 10.1016/s0001-706x(97)00037-5. [DOI] [PubMed] [Google Scholar]
- 6.Mercado R, Pablo Otto J, Perez M. Seasonal variation of intestinal protozoa infections in outpatients of the North Section of Santiago, Chile. 1995–1996. Bol Chil Parasitol. 1999;54:41–44. [PubMed] [Google Scholar]
- 7.Da Costa-Macedo LM, Machado-Silva JR, Rodrigues-Silva R, Oliveira LM, Vianna MS. Intestinal parasites in preschool children of the slum communities of the city of Rio de Janeiro, Brasil. Cad Saude Publica. 1998;14:851–855. doi: 10.1590/s0102-311x1998000400027. [DOI] [PubMed] [Google Scholar]
- 8.Kobayashi J, Vannachone B, Xeutvongsa A, Manivang K, Ogawa S, Sato Y, Pholsena K. Prevalence of intestinal parasitic infection among children in two villages in Lao PDR. Southeast Asian J Trop Med Public Health. 1996;27:562–565. [PubMed] [Google Scholar]
- 9.Shlim DR, Hoge CW, Rajah R, Scott RM, Pandy P, Echeverria P. Persistent high risk of diarrhoea among foreigners in Nepal during the first 2 years of residence. Clin Infect Dis. 1999;29:613–616. doi: 10.1086/598642. [DOI] [PubMed] [Google Scholar]
- 10.Saiman L, Aronson J, Zhou J et al. Prevalence of infectious diseases among internationally adopted children. Pediatrics. 2001;108:608–612. doi: 10.1542/peds.108.3.608. [DOI] [PubMed] [Google Scholar]
- 11.Magambo JK, Zeyhle E, Wachira TM. Prevalence of intestinal parasites among children in southern Sudan. East Afr Med J. 1998;75:288–290. [PubMed] [Google Scholar]
- 12.Julvez J, Bade MA, Lamotte M et al. Intestinal parasitic diseases in an urban environment in Sahel. A study in a district of Niamey, Niger. Bull Soc Pathol Exot. 1998;91:424–427. [PubMed] [Google Scholar]
- 13.Nimri LF. Prevalence of giardiasis among primary school children. Child Care Health Dev. 1994;20:231–237. doi: 10.1111/j.1365-2214.1994.tb00386.x. [DOI] [PubMed] [Google Scholar]
- 14.Araj GF, Abdul-Baki NY, Hamze MM, Alami SY, Nassif RE, Naboulsi MS. Prevalence and aetiology of intestinal parasites in Lebanon. J Med Liban. 1996;44:129–133. [PubMed] [Google Scholar]
- 15.Lopez CE, Dykes AC, Juranek DD et al. Waterborne giardiasis: a community wide outbreak of disease and a high rate of asymptomatic infection. Am J Epidemiol. 1980;112:495–507. doi: 10.1093/oxfordjournals.aje.a113019. [DOI] [PubMed] [Google Scholar]
- 16.Pickering LK, Woodward WE, Du Pont HL, Sullivan P. Occurrence of Giardia lamblia in children in day care centers. J Pediatr. 1984;104:522–526. doi: 10.1016/s0022-3476(84)80540-5. [DOI] [PubMed] [Google Scholar]
- 17.Centers for Disease Control. Giardiasis surveillance United States, 1992–1997. Morb Mortal Wkly Rep. 2000;49:1–13. [PubMed] [Google Scholar]
- 18.Greig JD, Michel P, Wilson JB et al. A descriptive analysis of giardiasis cases reported in Ontario 1990–1998. Can J Public Health. 2001;92:361–365. doi: 10.1007/BF03404980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Porter JD, Ragazzoni HP, Buchanon JD, Waskin HA, Juranek DD, Parkin WE. Giardia transmission in a swimming pool. Am J Publ Heath. 1988;78:659–662. doi: 10.2105/ajph.78.6.659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Navin TR, Juranek DD, Ford M, Minedew DJ, Lippy EC, Pollard RA. Case-control study of waterborne giardiasis in Reno, Nevada. Am J Epidemiol. 1985;122:269–275. doi: 10.1093/oxfordjournals.aje.a114098. [DOI] [PubMed] [Google Scholar]
- 21.Mintz ED, Hudson-Wragg M, Mshar P, Cartter ML, Hadler JL. Foodborne giardiasis in a corporate office setting. J Infect Dis. 1993;167:250–253. doi: 10.1093/infdis/167.1.250. [DOI] [PubMed] [Google Scholar]
- 22.Dennis DT, Smith RP, Welch JJ et al. Endemic giardiasis in New-Hampshire: a case-control study of environmental risks. J Infect Dis. 1993;167:1391–1395. doi: 10.1093/infdis/167.6.1391. [DOI] [PubMed] [Google Scholar]
- 23.Birkhead G, Vogt RL. Surveillance for endemic Giardia lamblia infection in Vermont. Am J Epidemiol. 1989;129:762–768. doi: 10.1093/oxfordjournals.aje.a115191. [DOI] [PubMed] [Google Scholar]
- 24.Chute CG, Smith RP, Baron JA. Risk factors for endemic giardiasis. Am J Publ Health. 1987;77:585–587. doi: 10.2105/ajph.77.5.585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fraser GG, Cooke KR. Endemic giardiasis and municipal water supply. Am J Publ Health. 1991;81:760–762. doi: 10.2105/ajph.81.6.760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stuart JM, Orr HJ, Warburton FG et al. Risk factors for sporadic giardiasis: a case-control study in southwestern England. Emerg Infect Dis. 2003;3:229–233. doi: 10.3201/eid0902.010488. [DOI] [PubMed] [Google Scholar]
- 27.Rodrigues L, Kirkwood BR. Case-control design in the study of common diseases: updates on the demise of the rare disease assumption and the choice of sampling scheme for controls. Int J Epidemiol. 1990;19:205–213. doi: 10.1093/ije/19.1.205. [DOI] [PubMed] [Google Scholar]
- 28.Hopkins RS, Juranek DD. Acute giardiasis: an improved clinical case definition for studies. Am J Epidemiol. 1991;133:402–407. doi: 10.1093/oxfordjournals.aje.a115894. [DOI] [PubMed] [Google Scholar]
- 29.Hill DR. Giardiasis. Issues in diagnosis and management. Infect Dis Clin North Am. 1993;7:503–525. [PubMed] [Google Scholar]
- 30.Hoque ME, Hope VT, Scragg R, Kjelistrom T, Lay-Yee R. Nappy handling and risk of giardiasis. Lancet. 2001;357:1017–1018. doi: 10.1016/S0140-6736(00)04251-3. [DOI] [PubMed] [Google Scholar]
- 31.Buret A, den Hollander N, Wallis PM, Befus D, Olson ME. Zoonotic potential of giardiasis in domestic ruminants. J Infect Dis. 1990;162:231–237. doi: 10.1093/infdis/162.1.231. [DOI] [PubMed] [Google Scholar]
- 32.Warburton AR, Jones PH, Bruce J. Zoonotic transmission of giardiasis: a case control study. Commun Dis Rep. 1994;4:R32–R36. [PubMed] [Google Scholar]
- 33.Thompson RCA, Hopkins RM, Homan WL. Nomenclature and genetic groupings of Giardia infecting mammals. Parasitology today. 2000;16:210–213. doi: 10.1016/s0169-4758(99)01624-5. [DOI] [PubMed] [Google Scholar]
- 34.Gray SF, Gunnel DJ, Peters TJ. Risk factors for giardiasis: a case-control study in Avon and Somerset. Epidemiol Infect. 1994;113:95–102. doi: 10.1017/s0950268800051505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Castelli F, Pezzoli C, Tomasoni L. Epidemiology of travelers’ diarrhoea. J Travel Med. 2001;8:S26–S30. doi: 10.1111/j.1708-8305.2001.tb00543.x. [DOI] [PubMed] [Google Scholar]
- 36.Gray SF, Rouse AR. Giardiasis – a cause of travelers’ diarrhoea. Commun Dis Rep. 1992;2:R45–R47. [PubMed] [Google Scholar]
- 37.Schlosser O, Grall D, Laurenceau MN. Intestinal parasite carriage in workers exposed to sewage. Eur J Epidemiol. 1999;15:261–265. doi: 10.1023/a:1007535426462. [DOI] [PubMed] [Google Scholar]
- 38.Hoque ME, Hope VT, Kjellstrom T, Scragg R, Lay-Yee R. Risk of giardiasis in Aucklanders: a case-control study. Int J Infect Dis. 2002;6:191–197. doi: 10.1016/s1201-9712(02)90110-4. [DOI] [PubMed] [Google Scholar]
- 39.Santamaria J, Toranzos GA. Enteric pathogens and soil: a short review. Int Microbiol. 2003;6:5–9. doi: 10.1007/s10123-003-0096-1. [DOI] [PubMed] [Google Scholar]
- 40.Marshall MM, Naumovitz D, Ortega Y, Sterling CR. Waterborne protozoan pathogens. Clin Microbiol Rev. 1997;10:67–85. doi: 10.1128/cmr.10.1.67. [DOI] [PMC free article] [PubMed] [Google Scholar]