SUMMARY
The aim of this study was to describe the natural history of HCV after 16 years of infection, in a cohort of individuals who acquired their infections on a known date in the United Kingdom. A total of 924 HCV-infected transfusion recipients (cases) and 475 anti-HCV negative transfusion recipients (controls) were eligible for inclusion in the study. Survival was compared between cases and controls to see if there was any excess mortality attributable to HCV. The results show that all-cause mortality was not significantly different between cases and controls (hazard ratio 1·17, 95% CI 0·92–1·49, P=0·21). However, the risk of death directly from liver disease was higher in cases than controls (hazard ratio 2·71, 95% CI 1·09–6·75, P=0·03). Nearly 30% of those HCV-infected cases who died directly from liver disease were known to have consumed excess alcohol.
INTRODUCTION
Hepatitis C (HCV) is a common cause of liver disease [1] and a major health problem worldwide [2]. The natural history of HCV is not fully described, and it was for this reason that a national cohort study of HCV was established in the United Kingdom [3, 4]. This study compared a large group of patients who had been infected with HCV by blood transfusion (cases) with a group of anti-HCV negative transfusion recipients (controls). All these recipients had been traced during the national HCV lookback progamme that was initiated by the Department of Health to help find recipients of potentially infected blood transfused prior to the introduction of routine donor testing for HCV [5]. Patients were recruited and followed up via the HCV National Register [3].
After the first decade of infection there was some evidence that all-cause mortality might be higher in cases [hazard ratio (HR) 1·41, 95% confidence interval (CI) 0·95–2·08, P=0·08] and the survival curves of the cases and controls were diverging [4]. If this divergence in mortality were to persist, then after 16 years a significant difference in survival would be expected. Cases were nearly six times more likely to have died from liver disease than controls, but this difference was not significant (P=0·10).
The aim of this study was to re-examine this cohort of patients, 6 years later, to see if there was now any excess mortality among individuals infected with HCV compared to transfusion recipients who are anti-HCV negative.
METHODS
Of the 996 HCV-infected transfusion recipients who had been traced during the UK lookback programme [3], 924 cases were eligible for inclusion in this study [4]. A total of 475 of the 536 recipients who were traced during the HCV lookback programme, but who tested negative for anti-HCV, were eligible for inclusion in the study as controls [4]. The characteristics and exclusion criteria for these cases and controls have already been reported [4]. For those still alive at the end of 2004, the mean time since transfusion by the end of that year was 16·2 years (range 13·3–25·6) for cases and 16·8 years (range 13·3–30·0) for controls. The mean age at transfusion was 43·6 years (range 0·0–87·2) and 41·5 years (range 0·0–84·5) for cases and controls respectively. Cases did not differ by age or sex from the controls [4]. At baseline, 859 of the 924 cases (93%) were HCV antibody positive; 65 were indeterminate for HCV antibodies (indeterminate was defined as testing positive or indeterminate for HCV antibodies on initial HCV EIA testing, and indeterminate on confirmatory recombinant immunoblot assay (RIBA) testing – all were HCV RNA negative). The baseline HCV RNA status was positive in 697 (75%), negative in 187 (20%), and unknown in 40 (4%) cases. By the end of 2004, 185 (20%) of the cases had received antiviral treatment for their infections, 606 (66%) were known not to have been treated, and 133 (14%) were of unknown treatment status.
Data were collected for cases and controls at the time of initial counselling during the HCV lookback programme and from death certification. All-cause and liver-related mortality were compared between cases and controls. Mortality was said to be ‘liver-related’ if there was any mention of HCV or liver disease on the death certificate. By reviewing the text of the death certificates, deaths were further classified into those where liver-related disease was likely to have directly caused death. This included certificates that mentioned hepatocellular carcinoma or end stage liver disease (varices, ascites, liver failure or hepatic encephalopathy) or where liver disease was coded as the underlying and only cause of death. In this analysis, death certificates where liver disease or hepatitis C were mentioned only as contributory factors were excluded, because they were considered to probably have been influenced by knowledge of the patient’s HCV status. This review and classification was undertaken by two medically qualified consultants – an epidemiologist (M.E.R.) and a hepatologist, blinded to HCV status.
Differences in baseline data at counselling between cases and controls were assessed using t tests for means or χ2 tests for proportions. In order to test for differences in survival between eligible cases and controls, Cox’s proportional hazards survival analysis was used with survival taken from the date of counselling to death with censoring at the end of 2004. Multivariable modelling allowed adjustment for differences between cases and controls according to factors, such as alcohol consumption, and tests for interactions between HCV status and other factors that affect survival, like age and sex.
This study was approved by the North Thames Multicentre Research Ethics Committee.
RESULTS
By the end of 2004, 255 out of 924 (27·6%) eligible cases had died (Fig. 1). Of the 255 deaths, 66 (25·9%) had a mention on the death certificate of one or more liver-related conditions: hepatocellular carcinoma (n=9), liver encephalopathy (n=2), portal hypertension (n=1), ascites (n=1), varices (n=1), hepatic failure (n=10), cirrhosis (n=22), liver disease (n=6), or chronic hepatitis/hepatitis C (n=51). Of these 66 deaths, only 34 were considered to have died directly from liver disease. Of these 34, for seven (20%) the underlying cause of death was not coded to any liver condition (two were coded to transfusion and five to other conditions). At death, these 34 cases had been infected for 11·6 years on average (range 5·6–18·0). Of these 34 cases, only three were known to be anti-HBc positive (none were known to be HBsAg positive). Four of the 34 mentioned alcohol on their death certificate, and for a further six cases excessive alcohol consumption was reported at counselling or during follow-up. As such, 29·4% of those patients who died as a direct result of their liver disease were known to have had excessive alcohol consumption. Thirty-two of the 66 liver-related deaths were judged not to have died as a direct result of their liver disease. These 32 cases died of heart disease (n=11), cancer (n=9), pneumonia (n=5), cerebral vascular accidents (n=2), septicaemia (n=2), and a variety of other causes (n=3), with HCV or liver disease simply mentioned on the death certificate.
Fig. 1.
Mortality amongst 924 eligible cases (▪) and 475 eligible controls (□).
Of the controls, 112 out of 475 (23·6%) had died by the end of 2004 (Fig. 1) and only six (5·4%) had any liver-related condition mentioned on the death certificate. These individuals died from hepatocellular carcinoma (n=2), and hepatic failure: (i) of unknown cause (n=1), (ii) associated with alcohol (n=1), (iii) following paracetamol overdose (n=1), and (iv) in association with sickle cell disease (n=1). All were judged to have died directly from their liver disease. Of the six controls who died directly from liver disease, all were known to be HCV ribonucleic acid negative and four anti-HBc negative (the results for the other two were not known). None had reported significant alcohol consumption at counselling.
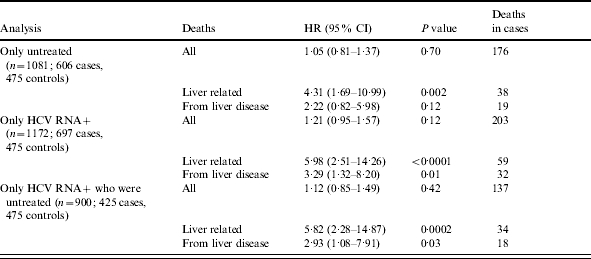
The survival analysis with all-cause mortality showed no evidence of a difference between cases and controls (HR 1·17, 95% CI 0·92–1·49, P=0·21) (Fig. 2). The proportional hazards assumption was checked by comparing the Kaplan–Meier survival curves with the fitted proportional hazards curves and also by testing the proportional hazard assumptions using Schoenfeld residuals (P=0·46). The significance of the case-control factor was also assessed using other survival models (Weibull and log-normal) and these gave similar results. Factors that significantly worsened survival were older age, being male, and level of alcohol consumption (all P<0·001, Table 1). Compared to drinkers of 1–20 units per week, survival was worse for cases with unknown (HR 2·77, 95% CI 1·96–3·92) and zero consumption (HR 1·51, 95% CI 1·17–1·96) and for those consuming  20 units per week (HR 1·15, 95% CI 0·80–1·64). There was no evidence that the relationship between survival and age, sex, or alcohol use differed between cases and controls. There was a significant difference in survival to death certified as liver-related (72 deaths: 66 cases, 6 controls) between cases and controls (HR 5·04, 95% CI 2·12–12·00, P=0·0003). Comparison of survival to a death directly from liver disease (40 deaths: 34 cases, 6 controls) was also statistically significant (HR 2·71, 95% CI 1·09–6·75, P=0·03). The three survival analyses, comparing survival to death from any cause, survival to death from a liver-related cause, and survival to death directly from liver disease were repeated for three subgroups of cases: first, restricting the analysis to those cases who had not been treated, second, restricting the analysis to those cases who were HCV RNA positive, and third, restricting the analysis to those cases who were PCR positive and who had not been treated. In none of these analyses was there any significant difference in survival between cases and controls (Table 2).
20 units per week (HR 1·15, 95% CI 0·80–1·64). There was no evidence that the relationship between survival and age, sex, or alcohol use differed between cases and controls. There was a significant difference in survival to death certified as liver-related (72 deaths: 66 cases, 6 controls) between cases and controls (HR 5·04, 95% CI 2·12–12·00, P=0·0003). Comparison of survival to a death directly from liver disease (40 deaths: 34 cases, 6 controls) was also statistically significant (HR 2·71, 95% CI 1·09–6·75, P=0·03). The three survival analyses, comparing survival to death from any cause, survival to death from a liver-related cause, and survival to death directly from liver disease were repeated for three subgroups of cases: first, restricting the analysis to those cases who had not been treated, second, restricting the analysis to those cases who were HCV RNA positive, and third, restricting the analysis to those cases who were PCR positive and who had not been treated. In none of these analyses was there any significant difference in survival between cases and controls (Table 2).
Fig. 2.
Kaplan–Meier survival curve for HCV cases and controls.
Table 1.
Survival (Cox’s proportional hazards model) multivariable analysis

HR, Hazard ratio; CI, confidence interval.
Table 2.
Survival (Cox’s proportional hazards model) multivariable sub-analyses
HR, Hazard ratio; CI, confidence interval.
DISCUSSION
After 16 years of infection, all-cause mortality among transfusion recipients who tested anti-HCV positive or indeterminate was 1·2 times greater than that observed in a similarly traced group of HCV-negative transfusion recipients. This increased risk was not statistically significant, even when analyses were restricted to those who were HCV RNA positive, or untreated. A significantly increased risk of dying with liver-related causes was observed among the cases, but this association is probably because individuals who are known to be HCV-positive are more likely to have HCV, chronic hepatitis or liver disease mentioned on their death certificates. When this analysis was restricted to cases who died directly from liver disease, the risk of dying was significantly greater for cases than controls (P=0·03). There are inherent limitations associated with studies relying on data from death certification and these have already been reported [4], nevertheless, when the full text of death certificates are reviewed these biases should be reduced. For example, 20% of the cases classified as dying directly from liver disease were not assigned to an underlying liver disease code. Researchers relying on the underlying cause of death codes should be aware of this limitation.
Amongst the cases, excessive alcohol consumption was known to be implicated in nearly one third of all deaths from liver disease, and it was not possible to rule out alcohol as a contributory factor from the others. Since survival was worst amongst those individuals whose alcohol consumption was not known/reported (Table 1), it is possible that poor reporting in the clinical setting has weakened the ability of our study to examine this co-factor [6]. Despite this, survival was worst in those whose consumption exceeded 20 units of alcohol per week, compared to individuals who consumed 1–20 units per week. As demonstrated by others, overall survival was also worse in those whose who were teetotal, suggesting that moderate levels of alcohol consumption have protective effects against a variety of other diseases [7, 8].
To fully determine the natural history of HCV, longer term cohort studies are required. In the United States, Seeff et al. followed-up a cohort of non-A, non-B hepatitis transfusion recipients who had been identified in the early 1970s and compared their mortality with a group of matched, transfused, controls from the same studies who did not have hepatitis [9]. This latter study shows that all-cause mortality, although high, was not significantly different between cases and controls after 25 years [10]. However, the relatively low level of liver-related mortality (<3%) was found to be significantly higher among cases (4·1%) than controls (1·3%, P=0·05). In the present study, we found similar levels of mortality from liver disease (2·9%) even though our study participants had been infected for a relatively shorter period of time, with a similar difference between cases (3·7%) and controls (1·3%, P=0·03).
Currently, few cohort studies have exceeded 25 years of follow-up and, therefore, critical information has not been available beyond this time point. The alternative sources of data on the natural history of HCV are obtained from retrospective studies of individuals who have presented with chronic symptomatic disease. These studies tend to overestimate HCV-related disease because they exclude individuals with mild or subclinical disease, those who have not been tested for HCV, and those who have resolved their infections. A 45-year follow-up has been achieved by one retrospective cohort study that used archived serum collected for other reasons in the 1940s and 1950s to test for anti-HCV and HCV RNA [11]. Although the results of that study may have been limited by the quality of the specimens following prolonged storage and the representativeness of the sample (predominantly male US military recruits), HCV-positive individuals in this study had low liver-related morbidity and mortality rates. Similar to those studies of women who were exposed to HCV-contaminated immunoglobulin [12, 13], the present study suggests that otherwise healthy HCV-positive persons might be at lower risk of progressive liver disease than was previously thought. Nevertheless after 16 years of infection, we have shown that transfusion recipients who tested positive or indeterminate for antibodies to HCV are at increased risk of dying from liver disease compared to anti-HCV negative transfusion recipients.
APPENDIX. The HCV National Register Steering Group
Dr Graeme Alexander (Senior Lecturer and Consultant Hepatologist, Addenbrooke’s Hospital, Cambridge), Mr Brian Gunson (Lay Representative, Non-Executive Director, St Albans and Harpenden Primary Care Trust, Hertfordshire), Dr Helen Harris (Clinical Scientist – Epidemiology, Health Protection Agency, London), Dr Julia Heptonstall (Consultant Microbiologist, Health Protection Agency, London), Dr Patricia Hewitt (Lead Consultant, National Blood Service, London), Professor Giorgina Mieli-Vergani (Consultant Paediatric Hepatologist and Director of Paediatric Liver Services, King’s College Hospital, London), Dr Hugh Nicholas (Senior Medical Officer, UK Department of Health, London), Professor Bernard Portmann (Consultant Histopathologist, Institute of Liver Studies, King’s College Hospital, London), Dr Mary Ramsay (Consultant Epidemiologist, Health Protection Agency, London), and Dr Angela Robinson (Medical Director, National Blood Authority, Watford).
ACKNOWLEDGEMENTS
We thank all the clinicians and research nurses who have supported this national project by enrolling their patients. We also thank Dr Graeme Alexander for his help reviewing the text of the death certificates. This research was funded by the UK Department of Health.
DECLARATION OF INTEREST
None.
REFERENCES
- 1.Seeff LB. The natural history of hepatitis C – a quandary. Hepatology. 1998;28:1710–1712. doi: 10.1002/hep.510280636. [DOI] [PubMed] [Google Scholar]
- 2.Anon. Hepatitis C – global prevalence (update) Wkly Epidemiol Rec. 1999;74:425–427. [PubMed] [Google Scholar]
- 3.Harris HE, Ramsay ME, Heptonstall J, Soldan K, Eldridge KP. HCV National Register Steering Group. The HCV National Register: towards informing the natural history of hepatitis C infection in the UK. J Viral Hep. 2000;7:420–427. doi: 10.1046/j.1365-2893.2000.00255.x. [DOI] [PubMed] [Google Scholar]
- 4.Harris HE, Ramsay ME, Andrews N, Eldridge KP. HCV National Register Steering Group. Clinical course of hepatitis C virus during the first decade of infection: cohort study. BMJ. 2002;324:450–453. doi: 10.1136/bmj.324.7335.450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chief Medical Officer. London: HMSO; 1995. Hepatitis C and blood transfusion lookback [PL CMO(95) 1] [Google Scholar]
- 6.Embree BG, Whitehead PC. Validity and reliability of self-reported drinking behavior: dealing with the problem of response bias. J Stud Alcohol. 1993;54:334–344. doi: 10.15288/jsa.1993.54.334. [DOI] [PubMed] [Google Scholar]
- 7.Yusuf S, Hawken S, Ounpuu S et al. Effect of potentially modifiable risk factors associated with myocardial infarction in 52 countries (the INTERHEART study): case-control study. Lancet. 2004;364:937–952. doi: 10.1016/S0140-6736(04)17018-9. [DOI] [PubMed] [Google Scholar]
- 8.Ruitenberg A, van Swieten J, Wittemann J et al. Alcohol consumption and risk of dementia: the Rotterdam Study. Lancet. 2002;359:281–286. doi: 10.1016/S0140-6736(02)07493-7. [DOI] [PubMed] [Google Scholar]
- 9.Seeff LB, Buskell-Bales Z, Wright EC et al. Long-term mortality after transfusion-associated non-A, non-B hepatitis. The National Heart, Lung and Blood Institute Study Group. New Engl J Med. 1992;327:1906–1911. doi: 10.1056/NEJM199212313272703. [DOI] [PubMed] [Google Scholar]
- 10.Seeff LB, Hollinger FB, Alter HJ et al. Long-term mortality and morbidity of transfusion-associated non-A, non-B and type C hepatitis: a National Heart, Lung, and Blood Institute collaborative study. Hepatology. 2001;33:455–463. doi: 10.1053/jhep.2001.21905. [DOI] [PubMed] [Google Scholar]
- 11.Seeff LB, Miller RN, Rabkin CS et al. 45-year follow-up of hepatitis C virus infection in healthy young adults. Ann Intern Med. 2000;132:105–111. doi: 10.7326/0003-4819-132-2-200001180-00003. [DOI] [PubMed] [Google Scholar]
- 12.Dittmann S, Roggendorf M, Durkop J, Wiese M, Lorbeer B, Deinhardt F. Long-term persistence of hepatitis C virus antibodies in a single source outbreak. J Hepatol. 1991;13:323–327. doi: 10.1016/0168-8278(91)90076-n. [DOI] [PubMed] [Google Scholar]
- 13.Kenny-Walsh E. Clinical outcomes after hepatitis C infection from contaminated anti-D immune globulin. Irish Hepatology Research Group. New Engl J Med. 1999;340:1228–1233. doi: 10.1056/NEJM199904223401602. [DOI] [PubMed] [Google Scholar]




