SUMMARY
Helicobacter pylori is transmitted within households and high concordance is observed among siblings. To better understand the contributions of close interpersonal contact and family relatedness to transmission, we compared concordance of H. pylori infection among 241 sibling and non-sibling children aged 2–18 years in 68, predominantly low-income, Hispanic households with at least two nuclear families. Prevalence of H. pylori infection was 24%. Compared to children with no infected siblings or non-siblings and adjusting for age, odds of H. pylori infection were 1·2 (95% CI 0·52–2·9), 3·2 (95% CI 1·14–9·1), and 9·4 (95% CI 3·1–28·5) for children residing with at least one infected non-sibling, one infected sibling, and with at least one infected sibling and non-sibling, respectively. The study further implicates intersibling transmission as a pathway for H. pylori infection in childhood. In addition, living with a non-sibling in extended-family homes may contribute to infection risk but only in households with prevalent H. pylori infection within all family groups.
INTRODUCTION
Helicobacter pylori, one of the most common chronic infections of the developing world and a known cause of gastric ulcers and cancer, appears to be acquired predominantly in childhood [1]. In industrialized countries, transmission has decreased in recent decades and only 10–20% of adults less than 30 years of age are infected [2] compared to 50% of the population above 60 years of age [3, 4]. The different prevalence by age reflects a ‘cohort’ phenomenon, i.e. decreasing risk of acquiring new infection over time. This decreased risk, in turn, is thought to reflect improvements in household sanitation and hygiene throughout the 20th century. Although H. pylori acquisition has been clearly associated with hygienic factors – such as bed sharing, household crowding, and lack of indoor plumbing – the exact route of transmission remains unclear [5–7].
Epidemiological data have suggested mechanisms consistent with person-to-person transmission. The organism has been cultured from vomitus [8], human faeces and saliva [9–13]. In addition, the many studies documenting clustering of fingerprinted organisms have supported transmission within families, finding infection rates among children to be significantly higher when their mothers were infected [14–20]. In addition, children may also have different strains from their parents [20]. H. pylori infection in young children has also been strongly linked to having infected siblings close in age [14, 17, 19, 21–24], and, particularly having infected older siblings [21].
The majority of evidence studying concordance of infection status among family members, particularly among children in the same age group, is consistent with person-to-person transmission, although exposure to common environmental sources has not been clearly eliminated [25, 26]. Concordance among family members, however, has also introduced the possible role of genetic susceptibility in influencing such transmission. Because studies of familial transmission include only participants sharing a strong genetic similarity, they are unable to clearly discriminate between the importance of close contact and that of genetics in person-to-person transmission. The only study to date that strongly suggested a genetic contribution to transmission identified higher concordance rates within the monozygotic than among dizygotic twin pairs [27].
A large ongoing trial of gastroenteritis transmission within Bay area households has provided us with an opportunity to explore concordance of H. pylori infection within complex households containing related and unrelated individuals. In these households, we examined concordance of infection among children of extended families (defined as at least two nuclear families) living together. By comparing infection rates among siblings and non-siblings living in the same home, we hope to better dissect the contributions of shared living and familial relatedness to H. pylori transmission.
METHODS
Study design and population
The Stanford Infection and Familial Transmission (SIFT) study is a prospective cohort study initiated in 1999 to assess H. pylori infection in association with household episodes of gastroenteritis [28]. Households in the Santa Clara and San Mateo counties of Northern California were recruited by community outreach as well as through 15 cooperating community health-care programmes, including general medicine outpatient clinics, emergency rooms, and paediatric clinics, and two county environmental health surveillance programmes that receive reports of gastroenteritis from the community.
Cases of diarrhoea and/or vomiting of suspected infectious aetiology (‘index case’) who presented at cooperating clinics were asked for permission to be contacted by study personnel. Those who consented to referral received a brief telephone interview to elicit study eligibility criteria, and, if appropriate, to schedule a home visit for which all interested household members were asked to be present. At each home visit informed consent was administered individually to all interested household members (‘participants’) and, for those consenting, a structured questionnaire regarding demographic characteristics, socio-economic markers, risk factors for H. pylori infection, household composition and family relationships was administered. Serum samples were collected for determination of H. pylori infection status. All visits were conducted by research staff with phlebotomy certification and fluent in the preferred language of the household. On average, ∼87% of all known household members have been available for interviews and ∼62% of those over age 2 years have participated in laboratory testing as well as interviews.
A household member was defined as someone who spent at least 20 h per week in the home and shared kitchen and bathroom facilities. As part of the home interview, participants were asked to identify distinct biological and/or economic family units within the home and the relationship of each household member to the index case (parent/spouse, child/sibling, aunt/uncle, niece/nephew, grandparent, child-care worker, unknown). Within family units, individuals were further identified as parent, offspring, other relative, or unknown. Households contained from 2–21 members and up to eight distinct family units. For the purposes of our current analysis, only households containing at least two distinct family units, each with at least one child between the ages of 2 and 18 years tested for H. pylori, were selected.
Laboratory methods
H. pylori infection was diagnosed by enzyme-linked immunosorbent assay (ELISA) for IgG using an assay validated in our laboratory, as previously described [29]. This assay uses high-molecular-weight, cell-associated proteins for five strains as antigen, including two Mexican strains. The sensitivity and specificity of this assay based on 77 persons from different ethnic groups were 94 and 91% respectively. Borderline results (∼4% of serology runs) were considered negative. Because serology may be less reliable in children <2 years of age, these children were excluded from the analysis.
Analytical methods
Children were defined as individuals between the ages of 2 and 18 years inclusive living in the household. For each child with H. pylori results, we examined the infection status of other children in the household. Children within the same family unit and of the same parents were considered siblings. Children within the same household of different families were considered related or unrelated non-siblings. For each child, the number of other siblings and/or non-siblings, as well as the number of infected siblings and/or infected non-siblings, was tallied.
We evaluated the likelihood of H. pylori infection given the number and infection status of other siblings and/or non-siblings in the home. Using children residing with no infected children as a reference, logistic regression was used to estimate the odds ratio (OR) and 95% confidence intervals (CIs) of infection among children residing with at least one infected non-sibling, at least one infected sibling, and with both an infected sibling and non-sibling. We also examined the effect of other risk factors, including age of child, household size, sleeping density, and highest educational attainment of adults in the home. To account for correlation of events within households, final models were fitted with a random intercept nonlinear mixed model (GLMMIX, SAS v. 9.0, SAS Institute, Cary, NC, USA), using household ID as the random effect. Because some children might not be at risk of sibling transmission due to absence of other siblings in the home, these analyses were repeated excluding children who did not live with at least one sibling. In addition, in order to inspect the redundancy of the logistic model, we used stratified random sampling with replacement to select one child per household and estimate 95% CIs in a bootstrap sample of 1500 replicates. Bootstrapped CIs were estimated using the percentile method. The bootstrap was also repeated using only children who had both a sibling and a non-sibling in the home.
RESULTS
Of a total of 1186 households enrolled in the study, 554 (47%) contained extended-family units (range 2–8), of which 369 (67%) included children between the ages of 2 and 18 years who participated in H. pylori testing. Of 369 extended-family households with children tested for H. pylori, 187 had at least two children tested for infection, of which 119 contained children in one family unit only, and 68 had at least one child in two different family units.
The 68 households included a total 638 participating members in 153 distinct family units, including 323 children <18 years of age. Of the 323 children, 241 (75%) were at least 2 years of age and tested for, H. pylori and included in the analysis. Of 82 children not included in the analysis, 58 who were <2 years, and 24 who were >2 years were not included because they did not participate in the home visit. Thus, the 241 children represented 91% of those eligible for analysis in these homes.
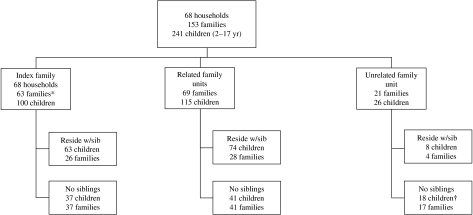
The social structure of these 68 households is summarized in the Figure, with the family of the index case denoted as the index family, and other family units defined as related or unrelated to the index unit. Of a total 241 children included in the analysis, 100 (41%) were members of the index unit, and 141 were members of other family units within the home, including 115 (82%) who were related to the index case, and 26 (18%) who were unrelated. Of these 26 children, only four were unrelated to each other. The 241 children resided with a median of three other children (range 1–9), including a median of one sibling (range 0–4) and two non-siblings (range 1–9). A total of 96 children (40%) resided with other (predominately related) non-sibling children only. Compared to SIFT children residing in single-family homes with at least two children, SIFT children living in extended-family households (Table 1) were more likely to be of Hispanic ethnicity but did not differ significantly in age or gender.
Fig.
Household composition according to index family. * Index refers to the criteria for selection for the SIFT study – i.e. gastroenteritis. In five index families the referring case was an adult with either no children aged 2–18 years or no children with available H. pylori results. These five households did have children aged 2–18 years tested in at least two other families in the household and, subsequently, eligible for inclusion into the study. † One family contained two children that were not biologically related and not considered siblings for the purposes of our study.
Table 1.
Characteristics of children aged 2–18 years residing with at least one other child in extended-family and single-family homes
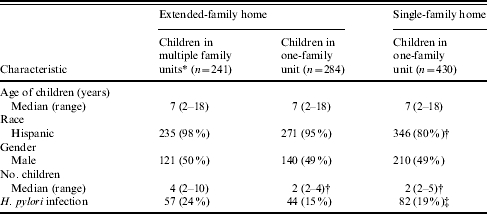
Children included in the analysis.
P<0·05 compared to 241 children included in the analysis.
P=0·06 for comparison of infection rate across three groups.
Compared to all other households participating in the SIFT study (Table 2), households of the children included in the analysis were larger (median 10 vs. 6 members, P<0·0001), including twice as many children, of greater sleeping density (median 3 vs. 2 persons/bedroom, P<0·0001), of lower educational attainment (12 vs. 11 years, P=0·02), and more likely to report Spanish as the primary language of the household (93% vs. 71%, P<0·0001). In addition, 88% of these households had at least one adult (⩾18 years of age) member who tested positive for infection, vs. 65% of other households (P<0·0001). Compared to all other extended-family homes, households included in the analysis were larger (median 10 vs. 7 members, P<0·001), with greater sleeping density (median 2·5 vs. 2 persons/bedroom, P<0·01), and more likely to have at least one adult infection (88% vs. 73%, P<0·01 respectively).
Table 2.
Household characteristics
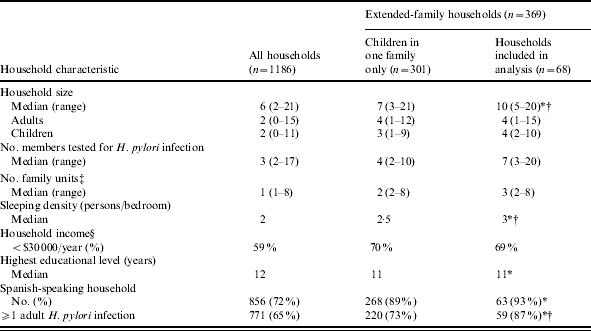
P<0·05 vs. 1118 households not included in the analysis.
P<0·05 vs. 301 households not included in the analysis.
A family unit was defined as a separate biological and/or economic unit within the household.
Based on 71% response rate.
H. pylori prevalence was 24% overall among study children, compared with 15 and 19% in predominantly sibling-only households, extended-family homes containing at least two children in one family unit and single-family homes with at least two children respectively (Table 1, P=0·06). Without accounting for infection status of other children, factors associated with H. pylori infection included age of the child (OR 2·0, 95% CI 1·4–2·8, per 5-year difference, P<0·0001), and total number of other siblings in the home (OR 1·4, 95% CI 1·1–1·8, per one sibling, P=0·02). The total number of other children in the household, total number of other non-siblings, gender, household educational attainment, and sleeping density of members within a household were not associated with H. pylori infection.
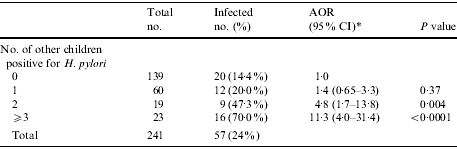
Prevalence of infection rose significantly with number of infected other children in the household (Table 3), including 14, 20, 47 and 70% of children residing with no infected children, with one, two, or three or more infected children respectively (χ2 test for trend, P<0·0001). Twelve ‘high transmission’ households – those homes with at least two infected children – accounted for nearly 65% of all H. pylori infections. Compared to children residing with no infected other children and controlling for age as well as household membership (Table 4), children residing with an infected sibling were significantly more likely to be infected themselves [adjusted odds ratio (AOR) 3·2, 95% CI 1·1–9·1], and children residing with at least one infected sibling and non-sibling were nine times more likely to be infected themselves (AOR 9·4, 95% CI 3·1–28·5). By contrast, children who resided only with infected non-siblings were not more likely than children residing with no infected children to be infected (AOR 1·22, 95% CI 0·52–2·9). Thus, the effect of living with infected non-siblings was significant only in concert with the presence of an infected sibling.
Table 3.
Frequency of H. pylori infection among 241 children in extended-family homes
AOR, Adjusted odds ratio; CI, confidence interval.
Adjusted for child’s age.
Table 4.
Odds of H. pylori infection accounting for infection status and familial relationship of other children in the household
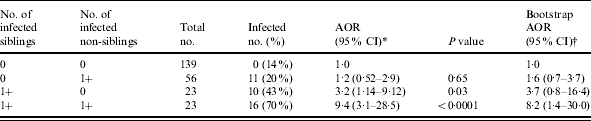
AOR, Adjusted odds ratio; CI, confidence interval.
Random effects model accounting for household membership (adjusted for age).
1500 replicates sampling one child per household (adjusted for age).
Excluding the 96 children who did not reside with a sibling (and thus had no chance of sibling transmission), the findings remained significant (Table 5). The odds of infection given at least one infected sibling was, adjusting for age (AOR 4·4, 95% CI 1·4–13·9), and for residing with at least one infected sibling and one infected non-sibling (AOR 14·3, 95% CI 4·5–45·8). In the 12 high-transmission households, 70% of children had at least one infected sibling.
Table 5.
Odds of H. pylori infection for only children living with at least one sibling and one non-sibling, accounting for infection status and familial relationship of other children in the household
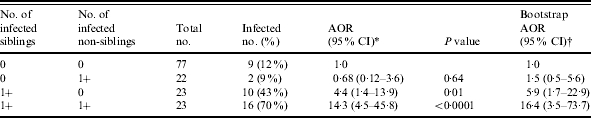
AOR, Adjusted odds ratio; CI, confidence interval.
Random effects model accounting for household membership (adjusted for age).
1000 replicates sampling one child per 45 extended-family households with at least one sibling and at least one non-sibling (adjusted for age).
Results of the bootstrap simulations (Tables 4 and 5) were largely corroborative of these results; however, as expected, CIs were wider, and only the category of children residing with both an infected sibling and non-sibling remained significant after adjustment for age.
DISCUSSION
H. pylori is one of the most common bacterial pathogens of humans, with considerable public health importance. Although evidence suggests infection is acquired primarily in early childhood [1], the epidemiology of childhood transmission has yet to be to be clearly elucidated. Despite well-established associations between H. pylori infection and crowded living conditions and strong evidence of familial concordance, knowledge of specific transmission pathways remains largely circumstantial. Studies delineating the social structures of high-risk households can help to clarify the social and familial pathways involved in H. pylori transmission.
In this study, we evaluated concordance of infection among children living in extended-family US immigrant homes. These predominately Hispanic households were crowded and complex, containing up to 10 related and unrelated children in up to eight different family units. Prevalence of H. pylori infection among children was 24%, and higher than infection rates estimated for the general population of US children. While household characteristics, including total number of other children in a household, were not associated with H. pylori infection; residing with two or more other children who were infected was highly associated with infection. In addition, when evaluated separately, residing with an infected sibling was more influential than residing with an infected non-sibling. At the same time, a significant additive effect of sibling and non-sibling status was also observed, i.e. children residing in households including at least one infected sibling and non-sibling were three times more likely than children residing with an infected sibling only to be concordant for infection. This suggests that the presence of infected non-siblings can play an important role in transmission in households with multiple infections among children. Transmission from infected non-siblings may occur less frequently than inter-sibling transmission, but in concert with infected siblings, risk of transmission may be modified importantly in crowded homes.
The importance of intra-familial transmission in H. pylori infection has been previously established [5, 30, 31]. Several studies have reported strong vertical (parent–child) associations, particularly between mother and child, probably reflecting the mother’s role as the primary caretaker in early childhood [14–20]. In crowded homes, however, it is not uncommon for children to share beds or for older children to participate in child-care responsibilities. Although not consistently as strong as vertical (parent–child) associations, more recent studies have suggested that close contact among siblings is also associated with household prevalence of infection among children [14, 17, 19, 21–24]. Among a paediatric Taiwanese population, children with an older seropositive sibling were nearly four times more likely than children with a seronegative older sibling to be infected [24]. A Brazilian study found that preschool children with a positive sibling had a nearly two-fold greater risk of infection [14]. In a study of rural Andean children aged 2–9 years, Goodman et al. reported a gradient effect, ranging from 1·7 to 7·1, for children with 1–4 positive siblings when compared to children with no infected siblings. The pattern of transmission was found strongest in those siblings close in age, particularly from older siblings to younger ones [21]. Our results expand on this research and also suggest that delineating familial relationships among cohabitating children in high-risk homes may yield further insight into the interaction of social and genetic risk factors for transmission.
H. pylori DNA strain analysis in families has also illustrated H. pylori transmission patterns among siblings as a predominant pathway [20, 30–33]. Over 80% of 36 families in a Swedish-based study had at least two siblings sharing H. pylori strain concordance. By comparison, mother and child concordance was only 50% in 18 families analysed [20]. A smaller Taiwanese study found strain concordance among the siblings in each of the five families studied whereas only two families revealed the same children sharing a strain of H. pylori with at least one of the parents [33]. Inter-generational strain segregation is consistent with the hypothesis of secular cohort effects in prevalence of H. pylori infection, including an important role for child-to-child transmission within households.
Siblings may play more closely together than non-siblings and may also have longer periods of cohabitation. Since non-siblings were predominately second-degree relatives and we did not collect information on duration of cohabitation, the higher concordance of infection among siblings than among non-siblings cannot be deemed in this study to implicate genetics in H. pylori transmission (although neither can we rule out this possibility). The lower risk conferred by living with infected non-siblings could suggest that higher thresholds of contact with infected, non-sibling children might be required for their presence to impact on incidence of infection. Of 42 children living in households with at least two other infected children, 83% were in an environment where at least half of the infected children were non-siblings. Therefore, our result was not simply masking the known significant effect infected siblings’ play in transmission.
Although intra-familial transmission has been accepted as a major factor for H. pylori infection the relative contributions of close interpersonal contact and genetic similarity to this pathway are not well understood. Our study is unique in that other studies directly comparing the effects of siblings and non-siblings in child-to-child transmission of H. pylori are not available. The impact on transmission by unrelated children is still unmeasured as H. pylori concordance studies of children in day-care centres are largely lacking. A study of Swedish schoolchildren aged 10–12 years found patterns of infection in children more closely correlated among their family members rather than their peers [34]. The study had limitations as there were obvious differences in degree of contact between the two groups and that family members, not solely siblings, was one comparison group. This study provides an initial analysis towards better understanding underlying factors for intra-familial transmission.
Some caveats about our study should be noted. The sampling design elicited households that are not typical of US homes, and even somewhat atypical of settled immigrant homes. Even within the context of our underlying cohort study, which includes many low-income Hispanic families, these homes were larger, with higher sleeping densities, of lower income, and of lower educational attainment – all factors linked with increased risk of H. pylori infection [5–7, 35–38]. Nonetheless, prevalence of H. pylori infection among children (24%) was not dissimilar to estimates for other US immigrant children [39–42]. Further, extended-family living arrangements are often a matter of convenience, and the composition of these homes can change frequently. Some arrangements in our study may have been temporary, and others more permanent, thus affecting the amount of time children are in close living situations with each other.
Although exposure to infected siblings appeared to play a significant role in explaining infection rates among children in these crowded households, 35 of the 68 households included in the analysis had no infected children and, ultimately, did not contribute to distinguishing the risk of transmission between sibling and non-sibling household contacts. Non-siblings in this study were usually second-degree relatives such as cousins, and the number of unrelated non-siblings was too small to distinguish these sources of non-sibling exposure. Only four children in 12 ‘high-transmission’ homes were found to be unrelated to the index child. Thus, it is unlikely that exposure to infected, unrelated non-siblings played a significant role in the results. While we cannot distinguish genetic from social explanations, sibling exposure was an independent predictor of infection when adjusted for sheer number of children, age, and other environmental factors such as household size or sleeping density.
Household concordance analysis involves complex clustering as well as redundancy effects. For this reason, we tested our results in a bootstrap simulation using replicate random samples of one child per household. While this analysis did widen CIs, conclusions were similar to the basic analysis for all households and those only restricted to having both siblings and non-siblings. Bootstrapping does not compensate for limitations of sample size. Although observed effect sizes, particularly for exposure to an infected sibling, were substantial, the modelling of interactions is a cautionary exercise with small numbers of children in some categories. In general, more complex statistical models are needed to delineate transmission problems where multiple interactions are of interest. Emerging methodological work in the area of network analysis may prove useful.
Our study further confirms child–child transmission as a likely pathway for H. pylori infection. That concordance was more common between siblings than non-siblings sharing a household may suggest a role for genetic similarity in transmission or that the contact required to transmit H. pylori must be at a high threshold to induce transmission. Further studies that are able to more fully account for duration of cohabitation and degree of contact can offer further clarification.
ACKNOWLEDGEMENTS
The authors thank Dr Bradley Efron and Dr Tyson Holmes of the Division of Statistics, Department of Health Research and Policy, Stanford University School of Medicine for their statistical support and critical review of the manuscript. This work was supported by: NIH R01 AI42801-05 (J. Parsonnet).
DECLARATION OF INTEREST
None.
Footnotes
Portions of this paper have been presented in preliminary form as Abstract #89920 at the American Public Health Association Conference, 6–10 November 2004, Washington, DC.
REFERENCES
- 1.Parsonnet J. The incidence of Helicobacter pylori infection. Aliment Pharmacol Ther. 1995;9:45–51. (Suppl 2): [PubMed] [Google Scholar]
- 2.Goodman KJ, Correa P, Tengana Aux HJ et al. Helicobacter pylori infection in the Colombian Andes: a population-based study of transmission pathways. Am J Epidemiol. 1996;144:290–299. doi: 10.1093/oxfordjournals.aje.a008924. [DOI] [PubMed] [Google Scholar]
- 3.Jones DM, Eldridge J, Fox AJ, Sethi P, Whorwell PJ. Antibody to the gastric campylobacter-like organism (‘Campylobacter pyloridis’) – clinical correlations and distribution in the normal population. J Med Microbiol. 1986;22:57–62. doi: 10.1099/00222615-22-1-57. [DOI] [PubMed] [Google Scholar]
- 4.Perez-Perez GI, Dworkin BM, Chodos JE, Blaser MJ. Campylobacter pylori antibodies in humans. Ann Intern Med. 1988;109:11–17. doi: 10.7326/0003-4819-109-1-11. [DOI] [PubMed] [Google Scholar]
- 5.Mendall MA, Goggin PM, Molineaux N et al. Childhood living conditions and Helicobacter pylori seropositivity in adult life [see comment] Lancet. 1992;339:896–897. doi: 10.1016/0140-6736(92)90931-r. [DOI] [PubMed] [Google Scholar]
- 6.Whitaker CJ, Dubiel AJ, Galpin OP. Social and geographical risk factors in Helicobacter pylori infection. Epidemiol Infect. 1993;111:63–70. doi: 10.1017/s0950268800056685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mitchell HM, Li YY, Hu PJ et al. Epidemiology of Helicobacter pylori in southern China: identification of early childhood as the critical period for acquisition. J Infect Dis. 1992;166:149–153. doi: 10.1093/infdis/166.1.149. [DOI] [PubMed] [Google Scholar]
- 8.Parsonnet J, Shmuely H, Haggerty T. Fecal and oral shedding of Helicobacter pylori from healthy infected adults. J Am Med Assoc. 1999;282:2240–2245. doi: 10.1001/jama.282.23.2240. [DOI] [PubMed] [Google Scholar]
- 9.Megraud F., Rathbone BJ, Heatley RV. Helicobacter pylori and gastroduodenal disease. Oxford: Blackwell Scientific Publications; 1992. Epidemiology of Helicobacter pylori infection; pp. 107–123. [Google Scholar]
- 10.Langenberg W, Rauws EA, Oudbier JH, Tytgat GN. Patient-to-patient transmission of Campylobacter pylori infection by fiberoptic gastroduodenoscopy and biopsy. J Infect Dis. 1990;161:507–511. doi: 10.1093/infdis/161.3.507. [DOI] [PubMed] [Google Scholar]
- 11.Miyaji H, Kohli Y, Azuma T et al. Endoscopic cross-infection with Helicobacter pylori. Lancet. 1995;345:464. [PubMed] [Google Scholar]
- 12.Thomas JE, Gibson GR, Darboe MK, Dale A, Weaver LT. Isolation of Helicobacter pylori from human faeces [see comment] Lancet. 1992;340:1194–1195. doi: 10.1016/0140-6736(92)92894-l. [DOI] [PubMed] [Google Scholar]
- 13.Krajden S, Fuksa M, Anderson J et al. Examination of human stomach biopsies, saliva, and dental plaque for Campylobacter pylori. J Clin Microbiol. 1989;27:1397–1398. doi: 10.1128/jcm.27.6.1397-1398.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rocha GA, Rocha AM, Silva LD et al. Transmission of Helicobacter pylori infection in families of preschool-aged children from Minas Gerais, Brazil. Trop Med Int Health. 2003;8:987–991. doi: 10.1046/j.1360-2276.2003.01121.x. [DOI] [PubMed] [Google Scholar]
- 15.Malaty HM, Kumagai T, Tanaka E et al. Evidence from a nine-year birth cohort study in Japan of transmission pathways of Helicobacter pylori infection. J Clin Microbiol. 2000;38:1971–1973. doi: 10.1128/jcm.38.5.1971-1973.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ma JL, You WC, Gail MH et al. Helicobacter pylori infection and mode of transmission in a population at high risk of stomach cancer. Int J Epidemiol. 1998;27:570–573. doi: 10.1093/ije/27.4.570. [DOI] [PubMed] [Google Scholar]
- 17.Rothenbacher D, Winkler M, Gonser T, Adler G, Brenner H. Role of infected parents in transmission of helicobacter pylori to their children. Pediatr Infect Dis J. 2002;21:674–679. doi: 10.1097/00006454-200207000-00014. [DOI] [PubMed] [Google Scholar]
- 18.Rothenbacher D, Bode G, Berg G et al. Helicobacter pylori among preschool children and their parents: evidence of parent–child transmission [see comment] J Infect Dis. 1999;179:398–402. doi: 10.1086/314595. [DOI] [PubMed] [Google Scholar]
- 19.Miyaji H, Azuma T, Ito S et al. Helicobacter pylori infection occurs via close contact with infected individuals in early childhood. J Gastroenterol Hepatol. 2000;15:257–262. doi: 10.1046/j.1440-1746.2000.02070.x. [DOI] [PubMed] [Google Scholar]
- 20.Kivi M, Tindberg Y, Sorberg M et al. Concordance of Helicobacter pylori strains within families. J Clin Microbiol. 2003;41:5604–5608. doi: 10.1128/JCM.41.12.5604-5608.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Goodman KJ, Correa P. Transmission of Helicobacter pylori among siblings [see comment] Lancet. 2000;355:358–362. doi: 10.1016/S0140-6736(99)05273-3. [DOI] [PubMed] [Google Scholar]
- 22.Rowland M, Kumar D, Daly L, O’Connor P, Vaughan D, Drumm B. Low rates of Helicobacter pylori reinfection in children. Gastroenterology. 1999;117:336–341. doi: 10.1053/gast.1999.0029900336. [DOI] [PubMed] [Google Scholar]
- 23.Drumm B, Perez-Perez GI, Blaser MJ, Sherman PM. Intrafamilial clustering of Helicobacter pylori infection. N Engl J Med. 1990;322:359–363. doi: 10.1056/NEJM199002083220603. [DOI] [PubMed] [Google Scholar]
- 24.Teh BH, Lin JT, Pan WH et al. Seroprevalence and associated risk factors of Helicobacter pylori infection in Taiwan. Anticancer Res. 1994;14:1389–1392. [PubMed] [Google Scholar]
- 25.Blecker U, Lanciers S, Mehta DI, Vandenplas Y. Familial clustering of Helicobacter pylori infection. Clin Pediatr. 1994;33:307–308. doi: 10.1177/000992289403300511. [DOI] [PubMed] [Google Scholar]
- 26.Webb PM, Knight T, Greaves S et al. Relation between infection with Helicobacter pylori and living conditions in childhood: evidence for person to person transmission in early life. BMJ. 1994;308:750–753. doi: 10.1136/bmj.308.6931.750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Malaty HM, Engstrand L, Pedersen NL, Graham DY. Helicobacter pylori infection: genetic and environmental influences. A study of twins [see comment]. Ann Intern Med. 1994;120:982–986. doi: 10.7326/0003-4819-120-12-199406150-00002. [DOI] [PubMed] [Google Scholar]
- 28.Perry S, Sanchez L, Yang S, Haggerty TD, Hurst P, Parsonnet J. Helicobacter pylori and risk of gastroenteritis. J Infect Dis. 2004;190:303–310. doi: 10.1086/421705. [DOI] [PubMed] [Google Scholar]
- 29.Replogle ML, Glaser SL, Hiatt RA, Parsonnet J. Biological sex as a risk factor for Helicobacter pylori infection in healthy young adults. Am J Epidemiol. 1995;142:856–863. doi: 10.1093/oxfordjournals.aje.a117725. [DOI] [PubMed] [Google Scholar]
- 30.Li L, Genta RM, Go MF, Gutierrez O, Kim JG, Graham DY. Helicobacter pylori strain and the pattern of gastritis among first-degree relatives of patients with gastric carcinoma. Helicobacter. 2002;7:349–355. doi: 10.1046/j.1523-5378.2002.00108.x. [DOI] [PubMed] [Google Scholar]
- 31.Miehlke S, Genta RM, Graham DY, Go MF. Molecular relationships of Helicobacter pylori strains in a family with gastroduodenal disease. Am J Gastroenterol. 1999;94:364–368. doi: 10.1111/j.1572-0241.1999.859_u.x. [DOI] [PubMed] [Google Scholar]
- 32.van der Ende A, Rauws EA, Feller M, Mulder CJ, Tytgat GN, Dankert J. Heterogeneous Helicobacter pylori isolates from members of a family with a history of peptic ulcer disease. Gastroenterology. 1996;111:638–647. doi: 10.1053/gast.1996.v111.pm8780568. [DOI] [PubMed] [Google Scholar]
- 33.Wang JT, Sheu JC, Lin JT, Wang TH, Wu MS. Direct DNA amplification and restriction pattern analysis of Helicobacter pylori in patients with duodenal ulcer and their families. J Infect Dis. 1993;168:1544–1548. doi: 10.1093/infdis/168.6.1544. [DOI] [PubMed] [Google Scholar]
- 34.Tindberg Y, Bengtsson C, Granath F, Blennow M, Nyren O, Granstrom M. Helicobacter pylori infection in Swedish school children: lack of evidence of child-to-child transmission outside the family [see comment] Gastroenterology. 2001;121:310–316. doi: 10.1053/gast.2001.26282. [DOI] [PubMed] [Google Scholar]
- 35.Dominici P, Bellentani S, Di Biase AR et al. Familial clustering of Helicobacter pylori infection: population based study [see comment] BMJ. 1999;319:537–540. doi: 10.1136/bmj.319.7209.537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Malaty HM, Graham DY. Importance of childhood socioeconomic status on the current prevalence of Helicobacter pylori infection. Gut. 1994;35:742–745. doi: 10.1136/gut.35.6.742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Replogle ML, Glaser SL, Hiatt RA, Parsonnet J. Biological sex as a risk factor for Helicobacter pylori infection in healthy young adults. Am J Epidemiol. 1995;142:856–863. doi: 10.1093/oxfordjournals.aje.a117725. [DOI] [PubMed] [Google Scholar]
- 38.Mitchell HM, Bohane TD, Tobias V et al. Helicobacter pylori infection in children: potential clues to pathogenesis [see comment] J Pediatr Gastroenterol Nutr. 1993;16:120–125. doi: 10.1097/00005176-199302000-00004. [DOI] [PubMed] [Google Scholar]
- 39.Megraud F, Brassens-Rabbe MP, Denis F, Belbouri A, Hoa DQ. Seroepidemiology of Campylobacter pylori infection in various populations. J Clin Microbiol. 1989;27:1870–1873. doi: 10.1128/jcm.27.8.1870-1873.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fiedorek SC, Malaty HM, Evans DL et al. Factors influencing the epidemiology of Helicobacter pylori infection in children. Pediatrics. 1991;88:578–582. [PubMed] [Google Scholar]
- 41.Crabtree JE, Mahony MJ, Taylor JD, Heatley RV, Littlewood JM, Tompkins DS. Immune responses to Helicobacter pylori in children with recurrent abdominal pain. J Clin Pathol. 1991;44:768–771. doi: 10.1136/jcp.44.9.768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Raymond J, Bergeret M, Benhamou PH, Mensah K, Dupont C. A 2-year study of Helicobacter pylori in children. J Clin Microbiol. 1994;32:461–463. doi: 10.1128/jcm.32.2.461-463.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]