SUMMARY
Since 1996 Salmonella Typhimurium DT104 salmonellosis has increased in The Netherlands. This prompted a case-control study of risk factors for salmonellosis to inform transmission routes for this phage type. Cases were laboratory-confirmed patients with a Salmonella infection and controls were selected from population registries by frequency matching for age, sex, degree of urbanization and season. Cases and controls received a questionnaire on risk factors. Of the 1171 cases, 573 (49%) responded: 245 S. Enteritidis and 232 S. Typhimurium cases (both DT104 and non-DT104), of which 58 were DT104. Of the 10 250 controls, 3409 (33%) responded. Use of H2 antagonists [odds ratio (OR) 4·4, 95% CI 1·6–12·2] and proton pump inhibitors (OR 4·2, 95% CI 2·2–7·9), consumption of raw eggs (OR 3·1, 95% CI 1·3–7·4) and products containing raw eggs (OR 1·8, 95% CI 1·1–3·0) were associated with endemic S. Enteritidis infection. Risk factors for endemic S. Typhimurium infection were use of proton pump inhibitors (OR 8·3, 95% CI 4·3–15·9), occupational exposure to raw meat (OR 3·0, 95% CI 1·1–7·9), playing in a sandbox (for children aged 4–12 years) (OR 2·4, 95% CI 1·6–3·7), consumption of undercooked meat (OR 2·2, 95% CI 1·1–4·1) and use of antibiotics (OR 1·9, 95% CI 1·0–3·4). Use of proton pump inhibitors (OR 11·2, 95% CI 3·9–31·9) and playing in a sandbox (OR 4·4, 95% CI 1·8–10·7) were the only risk factors for S. Typhimurium DT104 salmonellosis. This study confirms known risk factors for salmonellosis. However, playing in a sandbox was a predominant new risk factor for S. Typhimurium salmonellosis in children [population attributable risk (PAR) 14%], and especially for S. Typhimurium DT104 (PAR 32%).
INTRODUCTION
Annually, an estimated 4·5 million episodes of gastroenteritis occur in the Dutch population of 16 million. Around 50 000 cases each year are caused by Salmonella spp. [1]. At least one out of six cases consults a general practitioner [2–4], resulting in approximately 3700 laboratory-confirmed cases each year [4].
The predominant Salmonella serotype in The Netherlands is S. Enteritidis (40–45% of all isolates), followed by S. Typhimurium (around 30%) [5]. Although the incidence of salmonellosis has been decreasing, the emergence of S. Typhimurium DT104 as the principal phage type of S. Typhimurium since 1996 is worrisome, because of its multi-resistant character [4, 5–7]. In addition, these cases might be more severely ill than other S. Typhimurium or Enteritidis infections [8–10]. Phage type DT104 reached a peak in 2001 accounting for 15% of all Salmonella isolates and was 10 and 7% in 2002 and 2003, respectively [5, 11]. The emergence of DT104 also occurred in other European countries and the United States [12–14].
S. Enteritidis infections are mainly associated with consumption of poultry, eggs and egg-derived products [15–20], whereas S. Typhimurium can be found in a broad range of food products and DT104 has internationally been associated with consumption of beef, pork, chicken, raw milk and raw-milk cheese, travelling to foreign countries and contact with (farm) animals or a household member with gastroenteritis [5, 9, 10, 21–28].
In The Netherlands, S. Enteritidis is the predominant Salmonella serotype among layer chickens, but its prevalence is low among broilers. S. Typhimurium is found in cattle, pigs and sporadically in chicken [5, 29, 30], but transmission routes for human DT104 infections in The Netherlands are unclear. Therefore a case-control study on risk factors for salmonellosis was conducted, with special attention to S. Typhimurium DT104.
METHODS
A case-control study on risk factors for salmonellosis and campylobacteriosis was conducted from April 2002 to April 2003. This article is restricted to the Salmonella part of the study. Cases were laboratory-confirmed patients with a Salmonella infection, identified by examination of faecal samples submitted to the Regional Public Health Laboratories (RPHL) in The Netherlands, which cover about 62% of the Dutch population. All 17 laboratories participated, although one stopped in May, and another three only started in May, June and July 2002.
The RPHL sent the test result and a questionnaire and accompanying letter to the physician, who forwarded these to the patient. Salmonella isolates were sent to the National Institute of Public Health and the Environment for serotyping and phage typing as described previously [30] and to the Animal Sciences Group, Lelystad, The Netherlands, for antibiotic susceptibility testing. Based on historic surveillance of the numbers of confirmed Salmonella and Campylobacter cases in the RPHL, the expected numbers of cases by age, sex, degree of urbanization and season were obtained. Controls (aiming at two per case) were selected from the population registries of 25 municipalities within the service area of the RPHL by frequency matching for the above variables; 10 250 controls were approached in anticipation of an expected response rate of 25%.
The questionnaires for cases and controls included questions regarding food consumption, kitchen hygiene and food processing, contact with animals, occupational exposure, travel, water recreation, use of medication (during the previous 4 weeks) and contact with persons with gastroenteritis symptoms. Cases were also questioned about clinical symptoms and treatment. Questions covered the 7 days prior to symptom onset (cases) or completion of the questionnaire (controls). Parents were asked to complete questionnaires for young children.
The incidence of laboratory-confirmed S. Typhimurium and S. Enteritidis infection was calculated using the number of cases identified from the RPHL divided by the population covered by these laboratories. Adjustments in the denominator were made for the time each laboratory participated and for underreporting by the laboratories. The latter was based on a comparison between the reported number of Salmonella cases in this study and the regular laboratory surveillance of Salmonella: an overlap of 79% was found. From recognized outbreaks, all cases except one were excluded.
Missing values were handled using multiple imputation [31]. This method is recommended to avoid bias in analyses of epidemiological data with missing covariates [32]. Imputation was carried out with MICE [33]. In brief, five separate values for a missing data item were drawn from imputation models in which the value of this variable is predicted based on other variables in the dataset. We used linear imputation models including all variables that correlated higher than 0·1 with the variable to be imputed, and used predictive mean matching to draw imputed values from this model. This drawing method takes as an imputed value the observed value of the person for which the prediction model predicts a value closest to the predicted value of the person with the missing value. The parameters of the imputation model are established using Gibbs sampling, and thus differ between each of the five imputations so that different matches are usually made. This method assures that imputed values follow the same distribution as the observed values. With the five imputed datasets, five different logistic regressions (or other analyses) were carried out and the five results were pooled using SAS proc mianalyse in order to obtain a single final result.
For the risk analysis, cases with a Salmonella and a Campylobacter infection were excluded. Analyses were done using cross tabulations, χ2 tests and ‘single’ variable logistic regression models (which also included age, sex, degree of urbanization and level of education) for significance testing. For further analyses, only cases and controls who had not travelled abroad were included. Risk factors in the single-variable analyses (P⩽0·10) were selected for inclusion in a multivariate logistic regression model. The degree of urbanization was categorized by addresses per km2 as urban (>2500), urbanized (500–2500) and rural (<500). The level of education achieved by adult cases and controls, or by one of the parents for those under 18 years was classified as low (primary, lower vocational or lower secondary education), intermediate (intermediate vocational, intermediate secondary or higher secondary education), and high (higher vocational and university education).
The population attributable risk (PAR) of each risk factor was calculated based on multivariate odds ratios (ORs) as the number of cases attributable to the risk factor divided by the total number of cases. The number of cases attributable to the risk factor was calculated as the total number of cases minus the estimated number of cases if the risk factor was absent, which was estimated by weighting the cases according to their exposure status. Exposed cases were weighted as 1/OR of exposure, non-exposed cases as 1.
RESULTS
The RPHL identified 1194 cases with salmonellosis, of which 533 (45%) were S. Enteritidis and 437 (37%) were S. Typhimurium (10% DT104 and 27% non-DT104) cases. The overall incidences of S. Enteritidis and S. Typhimurium salmonellosis were 7 and 6 per 100 000 person-years respectively, including travel-related cases. The incidences of S. Typhimurium non-DT104 and DT104 were 4 and 1 per 100 000 person-years respectively (Table 1). The incidences of S. Enteritidis and S. Typhimurium were clearly higher for children aged 0–4 years and, to a lesser extent, children aged 5–17 years, and during the summer. For S. Typhimurium higher incidences were found in rural areas. For DT104 higher incidences were found during a longer period, from April to September (Table 1).
Table 1.
Incidence of S. Enteritidis and S. Typhimurium (total, and specific for DT104 and non-DT104) salmonellosis per 100 000 person-years by demographic variables, including travel-related cases. The Netherlands, April 2002 to April 2003
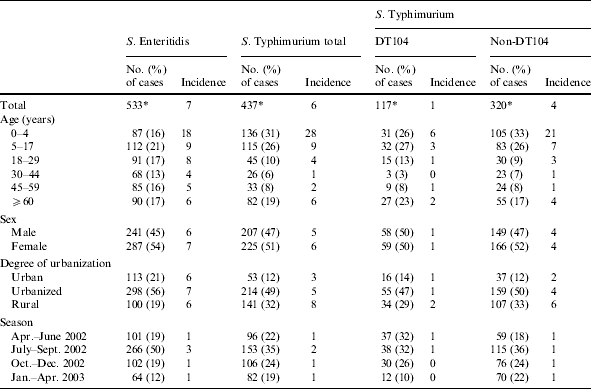
Totals do not always add up because of missing values.
Of the S. Enteritidis and S. Typhimurium cases respectively 245 (47%) and 232 (53%) completed a questionnaire; 174 (54%) of the S. Typhimurium non-DT104 cases and 58 (50%) of the DT104 cases responded. The majority of the cases reported diarrhoea (97%), abdominal cramps (79%), stomach ache (78%), fever (72%), mucus in the stool (60%) and nausea (53%), whereas 46% reported vomiting and 39% blood in the stool. The presence of blood in the stool was more frequently reported by S. Typhimurium cases than S. Enteritidis cases (46 and 32% respectively; χ2 test, P=0·005). Blood in the stool was also more frequent among DT104 cases than non-DT104 cases (55% vs. 43%; χ2 test, P=0·06), whereas having mucus in the stool was more often reported by non-DT104 cases (70% vs. 58%; χ2 test, P=0·11). Vomiting was more frequently associated with S. Typhimurium DT104 than non-DT104 (55 and 42% respectively; χ2 test, P=0·08).
At the time the questionnaire was completed, 77% of the S. Enteritidis and 67% of the S. Typhimurium cases had recovered; for DT104 and non-DT104 cases respectively, 71% and 63% had recovered at this time. The median duration of symptoms for recovered cases was 10–11 days for S. Enteritidis and S. Typhimurium cases [25th–75th percentile (P25–75): 7–14]. The median duration of symptoms was longer for DT104 than for non-DT104 infections: 14 days (P25–75: 7–17) vs. 10 days (P25–75: 7–14) (Kruskal–Wallis test, P=0·05). Of the cases that had not yet recovered, the median time between symptom onset and completion of the questionnaire was 19 days for S. Enteritidis infections (P25–75: 13–27) and 17 days for S. Typhimurium infections (P25–75: 12–25). This time interval was 14 days (P25–75: 10–34) and 17 days (P25–75: 12–25) for DT104 and non-DT104 respectively. Of the S. Enteritidis and S. Typhimurium cases, 48 (20%) and 50 (22%) respectively, were admitted to the hospital; 11 (19%) of the DT104 cases and 39 (22%) of the non-DT104 cases were hospitalized.
In total 10 250 controls were approached and 3409 (33%) completed the questionnaire. Travelling abroad within 7 days prior to symptom onset was reported by 77 S. Enteritidis (31%) and 34 S. Typhimurium cases (15%). Eight (14%) of the DT104 cases and 26 (15%) of the non-DT104 cases had travelled. Of the 3409 controls 244 (7%) reported travelling abroad within 7 days prior to completion of the questionnaire. In a single-variable analysis, foreign travel was strongly associated with salmonellosis (S. Enteritidis: OR 6·3, 95% CI 4·6–8·6; PAR 27%, 95% CI 25–28%; S. Typhimurium: OR 2·7, 95% CI 1·8–4·0; PAR 9%, 95% CI 7–11%; non-DT104: OR 2·8, 95% CI 1·8–4·4; PAR 10%, 95% CI 7–12%; DT104: OR 2·2, 95% CI 1·0–4·9; PAR 8%, 95% CI 0·4–11%).
For endemic Salmonella cases, the median time between symptom onset and completion of the questionnaire was 18 days (P25–75: 13–27). Consumption of raw eggs and products containing raw eggs and recent use of proton pump inhibitors and H2 antagonists were associated with endemic S. Enteritidis infections (Table 2). The PAR was highest for use of proton pump inhibitors (7%), followed by consumption of products containing raw eggs (5%), consumption of raw eggs (4%) and use of H2 antagonists (2%) (Table 2). Some exposures were negatively associated with S. Enteritidis salmonellosis: consumption of pork, sausage, fish, cheese, dairy products other than milk and cheese, non-homemade sandwich, raw and stir-fried vegetables, fruit with skin, chocolate and nuts. No associations were found for consumption of other food products, occupational exposure, ownership of (farm) animals and playing in a sandbox.
Table 2.
Single-variable and multivariate logistic regression analyses and population attributable risk of risk factors associated with endemic S. Enteritidis salmonellosis. A case-control study in The Netherlands, April 2002 to April 2003
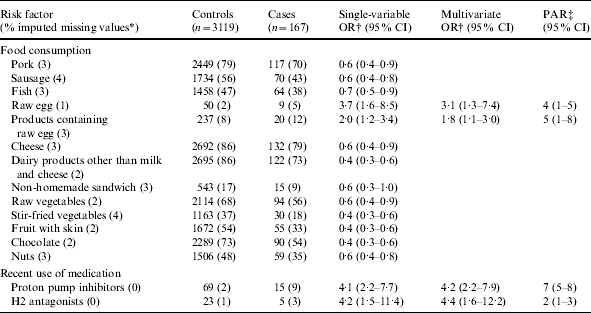
OR, Odds ratio; CI, confidence interval; PAR, population attributable risk.
Fraction of imputed missing values among cases and controls together.
Adjusted for age, sex, degree of urbanization and level of education.
Based on the multivariate odds ratio.
Consumption of undercooked meat, occupational exposure to raw meat and recent use of antibiotics and proton pump inhibitors were associated with endemic S. Typhimurium enteritis (Table 3). Of the 65 S. Typhimurium cases aged 4–12 years, 46 (71%) reported having played in a sandbox, while of the 374 controls aged 4–12 years, only 183 (49%) played in a sandbox (OR 2·5, 95% CI 1·4–4·7). In a multivariate model including consumption of undercooked meat, occupational exposure to raw meat, use of antibiotics and proton pump inhibitors and playing in a sandbox for children aged 4–12 years, these risk factors remained associated with S. Typhimurium salmonellosis (Table 4). The PAR was highest for playing in a sandbox for children aged 4–12 years (14%), followed by use of proton pump inhibitors (8%), consumption of undercooked meat (7%), use of antibiotics (4%) and occupational exposure to raw meat (2%). No association was found with playing in a sandbox for children aged 0–3 years. Many exposures were negatively associated with S. Typhimurium infection: consumption of chicken, beef, meat in paste, fish, pasteurized milk, cheese, dairy products other than milk and cheese, raw and stir-fried vegetables, fruit with skin, homemade sauce and nuts (Table 3). No associations were found for consumption of other food products, occupational exposure or ownership of (farm) animals.
Table 3.
Single-variable logistic regression analyses of risk factors associated with endemic S. Typhimurium salmonellosis, with distinction between DT104 and non-DT104. A case-control study in The Netherlands, April 2002 to April 2003
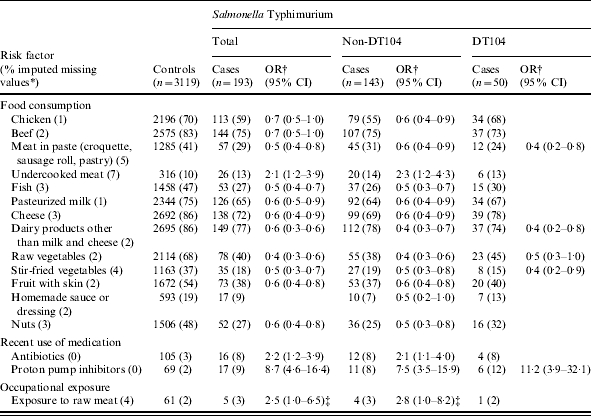
OR, Odds ratio; CI, confidence interval.
Fraction of imputed missing values among S. Typhimurium cases (total) and controls together.
Adjusted for age, sex, degree of urbanization and level of education.
Marginally significant: P=0·07 S. Typhimurium (total); P=0·06 S. Typhimurium non-DT104.
Table 4.
Multivariate logistic regression analyses and population attributable risk of risk factors associated with endemic S. Typhimurium salmonellosis, with distinction between DT104 and non-DT104. A case-control study in The Netherlands, April 2002 to April 2003
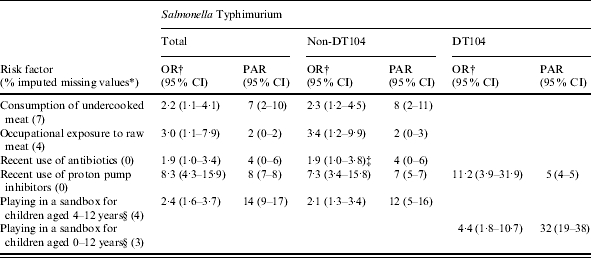
OR, Odds ratio; CI, confidence interval; PAR, population attributable risk.
Fraction of imputed missing values among S. Typhimurium (total) cases and controls together.
Adjusted for age, sex, degree of urbanization and level of education.
Reference category: cases and controls of a different age and children of the same age who did not play in a sandbox.
Marginally significant, P=0·058.
The recent use of proton pump inhibitors was also associated with endemic S. Typhimurium DT104 (Table 3). Moreover, 21 (78%) of the 27 DT104 cases aged 0–12 years played in a sandbox, whereas only 406 (54%) of the 757 controls aged 0–12 years played in a sandbox, yielding an OR of 3·0 (95% CI 1·2–7·5). In a multivariate model, use of proton pump inhibitors and playing in a sandbox for children aged 0–12 years remained associated with S. Typhimurium DT104 (Table 4). The PAR for playing in a sandbox was 32% and for use of proton pump inhibitors, 5%.
DISCUSSION
This is the first case-control study of risk factors for sporadic S. Enteritidis and S. Typhimurium enteritis in The Netherlands. Extrapolation of the incidences of these two serotypes in salmonellosis found in this study, according to the Dutch population at 1 January 2003, yields an estimate of respectively 1133 and 971 laboratory-confirmed cases and 16 255 and 13 931 community cases per year [1].
For S. Enteritidis infection, the association with (raw) eggs has been reported extensively in the literature [16–21]. In The Netherlands, the total number of Salmonella cases has decreased since 1996 (except for 2003) [4], but the relative contribution of eggs remained stable (∼35%). The absolute number of egg-associated cases also hardly declined after 1999 [5]. The current study confirms that S. Enteritidis is mainly attributable to (raw) eggs, however, the PAR calculated here was low. Possibly some cases might not have been aware that they ate foods prepared with raw eggs, leading to some misclassification with regard to this. Moreover, cross-contamination might play a role in kitchens where raw eggs were prepared (even if the eggs were not consumed raw), i.e. contamination of kitchen surfaces and utensils and hence other food items, not properly heated afterwards such as salads. This was not explicitly included in our questionnaire, and thus might have underestimated the PAR for raw eggs. Therefore, continued public and professional education about proper heating of eggs, minimizing cross-contamination and the risk of using raw eggs in foods not properly heated before consumption, is necessary. Ideally, food safety education should be supported by regulation. In October 2001, the Dutch Ministries of Agriculture and of Public Health announced legislation for a ban of shell eggs containing Salmonella in The Netherlands, but the draft proposal was turned down by the European Commission in 2003. Nevertheless, several hygiene codes in The Netherlands outline the use of pasteurized eggs instead of raw eggs in foods that are not properly heated before consumption.
S. Typhimurium was associated with consumption of undercooked meat and occupational exposure to raw meat. This corresponds with the high prevalence of S. Typhimurium in cattle and pigs [29, 30], and with outbreak reports of this serotype related to beef and pork [10, 24, 25]. However, the most important risk factor was newly identified: 44% of the S. Typhimurium cases aged 4–12 years, and 52% of the S. Typhimurium DT104 cases aged 0–12 years were attributable to playing in a sandbox. Considering all S. Typhimurium and DT104 cases specifically, 14 and 32% respectively, were attributable to this factor. This indicates that S. Typhimurium is not only confined to foods, but may be widespread in the environment or be more capable of infecting people upon contact than S. Enteritidis. The former hypothesis is supported by the almost three times higher incidence of S. Typhimurium in rural compared to urban areas. In a study of the home environment and salmonellosis in children, various Salmonella serotypes were found in the home environment, including soil, vacuum cleaners and pets [34]. A review of literature on waste disposal practices also showed that soil is a possible vector and reservoir of enteric pathogens [35]. Possibly, free-living soil nematodes, such as Caenorhabditis elegans that are known to harbour and disperse bacteria, including S. Typhimurium, may play a role in the spread and transmission of foodborne pathogens in soil [36].
However, sandboxes may not only show the ubiquitous occurrence of S. Typhimurium, but may also pose a ‘true’ risk for salmonellosis. Therefore, microbiological investigation of sandboxes is needed, with special attention to S. Typhimurium DT104. In The Netherlands, guidelines exist for sandboxes in public areas, recommending covering the sandbox, removing gross contamination and replacing sand when contaminated with animal faeces and refreshing the sand at least once a year. To reduce the risk for salmonellosis, such guidelines should also be brought to the attention of parents with young children and owners of private sandboxes by public education.
The recent use of H2 antagonists, proton pump inhibitors and antibiotics have previously been identified as risk factors [21, 23, 26, 37, 38]. The neutralization of gastric acid by anti-secretory drugs inhibits the protective antibacterial action of gastric juice and thus might facilitate Salmonella (and other) infections. Antimicrobial exposure might increase the susceptibility to Salmonella by altering the colonic bacterial flora, or receiving medications such as antibiotics might just be a marker of increased susceptibility to infections in general [23]. In addition, antimicrobial exposure might lead to direct selection of resistant strains, such as S. Typhimurium DT104, when using antibiotics to which the strain is resistant. Health-care providers and the public should be educated about the risk of infection when using anti-secretory drugs and antibiotics.
Some exposures were negatively associated with salmonellosis, among others, consumption of cheese, vegetables, fruit, chocolate and nuts. However, these factors may not be protective for salmonellosis, for it is not known if controls were actually exposed to the pathogen. A true protective effect should also be biologically plausible. In addition, mathematical models showed that negative associations can be found for risk factors when multiple exposures occur over many years, inducing partial immunity and less clinical illness [39].
A limitation of this study is the relatively low numbers of S. Typhimurium DT104 cases, which was insufficient to detect small differences in exposures between cases and controls. Furthermore, the recall period for all cases was longer than for controls, because for controls, questions addressed the 7 days before completion of the questionnaire, while for cases questions addressed the 7 days prior to symptom onset, which was a median 18 days before completion of the questionnaire. Multiple imputation was used for handling missing values. Before using this statistical method, in several questions, the ‘I don’t know’ category was associated with salmonellosis. This suggests that controls were better able to remember exposures than cases and consequently, recall bias could theoretically play a role. However, after using multiple imputation the results of the analysis were similar. This implies that missing values were randomly distributed over the response categories and were not dependent on a certain exposure status. The association between salmonellosis and consumption of (raw) eggs or undercooked meat is well known by the general public, which may lead to recall bias. However, the existence of different Salmonella serotypes is not generally known. As the association with raw eggs was specific for S. Enteritidis and the association with undercooked meat was specific for S. Typhimurium, recall bias is considered unlikely.
The present study included only laboratory-confirmed cases and, thus, reflects those with more severe symptoms or an illness of a longer duration [21]. Focusing on laboratory-confirmed cases might also overestimate the proportion of young children and travel-related cases, as these are more likely to consult a general practitioner and general practitioners are more likely to culture these cases [21, 40]. Therefore, the proportion of travel-associated S. Enteritidis, Typhimurium and Typhimurium DT104 cases, and the respective PARs of 27, 9 and 8% should be considered maximum values.
In conclusion, this study showed that S. Enteritidis salmonellosis is still mainly associated with consumption of (raw) eggs, but S. Typhimurium is associated with playing in a sandbox, next to consumption of undercooked meat and occupational exposure to raw meat. Public and professional education about proper food handling is needed. Ideally, banning shell eggs with Salmonella would reduce the incidence of S. Enteritidis considerably. For S. Typhimurium infections, measures to reduce the contamination of sand should be developed. Furthermore, public and professional education is needed to stress the importance of appropriate use of antibiotics and anti-secretory drugs, because these drugs facilitate Salmonella (and other) infections. Finally, elucidation of the role of immunity following an infection would importantly help to interpret results on risk factors and apparently protective factors and to judge the effect of control strategies.
ACKNOWLEDGEMENTS
We are most grateful to Dr M. A. S. de Wit for her contribution to the design of the study, and to the participating Regional Public Health Laboratories (RPHL) for their contribution to the data collection, especially Dr F. Vlaspolder, Dr J. H. Sloos, Dr J. Spaargaren, Dr J. Peereboom, Dr M. A. Schouten, Dr R. W. Brimicombe, Dr F. W. Sebens, Dr Ph. H. Rothbarth, Dr L. J. M. Sabbe, Dr H. Mulder, Dr Veenendaal, Dr E. Ijzerman, Dr J. H. T. Wagenvoort, Dr J. H. van Zeijl, Dr B. M. de Jongh, Dr M. Tersmette, Dr P. Voorn, Dr A. M. Horrevorts, Dr J. Buitenwerf, Dr B. G. A. Hendrickx, Dr M. Peeters and Dr A. R. Jansz. We also are grateful to H. M. E. Maas, A. J. Verbruggen and M. E. O. C. Heck for serotyping and phage typing of the Salmonella isolates. Furthermore, we thank Dr H. C. Boshuizen for her help and advice in the data analysis.
DECLARATION OF INTEREST
None.
REFERENCES
- 1.Wit MAS de, Koopmans MPG, Kortbeek LM et al. Sensor, a population-based cohort study on gastroenteritis in the Netherlands, incidence and etiology. Am J Epidemiol. 2001;154:666–674. doi: 10.1093/aje/154.7.666. [DOI] [PubMed] [Google Scholar]
- 2.Wit MAS de, Koopmans MPG, Kortbeek LM, Leeuwen WJ van, Bartelds AIM, Duynhoven YTHP van. Gastroenteritis in Sentinel General Practices, the Netherlands. Emerg Infect Dis. 2001;7:82–91. doi: 10.3201/eid0701.010113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wit MAS de, Kortbeek LM, Koopmans MPG et al. Comparison of gastroenteritis cases in a general practice-based study and a community-based study. Epidemiol Infect. 2001;127:389–397. doi: 10.1017/s0950268801006082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pelt W van, Wit MAS de, Wannet WJB, Ligtvoet EJJ, Widdowson MA, Duynhoven YTHP van. Laboratory surveillance of bacterial gastroenteric pathogens in The Netherlands, 1991–2001. Epidemiol Infect. 2003;130:431–434. [PMC free article] [PubMed] [Google Scholar]
- 5.Valkenburgh SM, Oosterom RAA van, Meijer-Leegwater MPM, Komijn RE, Pelt W van. 2004. Report on trends and sources of zoonotic agents, the Netherlands 2003. Ministry of Public Health, Welfare and Sport. Annual report according to article 5 of the Directive 92/117/EC. , June .
- 6.Pelt W van, Wit MAS de, Giessen AW van de, Leeuwen WJ van, Duynhoven YTPH van. Decrease of infections with Salmonella spp. in humans; demographic changes and shifts in serovars [in Dutch] Infectieziekten Bull. 1999;10:98–101. [Google Scholar]
- 7.Pelt W van, Min J, Veling J et al. An explosive increase of multiresistant Salmonella Typhimurium DT104 in 2001 in the Netherlands [in Dutch] Infectieziekten Bull. 2000;12:356–361. [Google Scholar]
- 8.Helms M, Vastrup P, Gerner-Smidt P, Mølbak K. Excess mortality associated with antimicrobial drug-resistant Salmonella Typhimurium. Emerg Infect Dis. 2002;8:490–495. doi: 10.3201/eid0805.010267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wall PG, Morgan D, Lamden K et al. A case control study of infection with an epidemic strain of multi-resistant Salmonella Typhimurium DT104 in England and Wales. Comm Dis Rep. 1994;4:R130–R135. [PubMed] [Google Scholar]
- 10.M⊘lbak K, Baggesen DL, Aarestrup FM et al. An outbreak of multidrug-resistant, quinolone-resistant Salmonella enterica serotype Typhimurium DT104. N Engl J Med. 1999;341:1420–1425. doi: 10.1056/NEJM199911043411902. [DOI] [PubMed] [Google Scholar]
- 11.Pelt W van, Wannet WJB, Giessen AW van de, Duynhoven YTHP van. Trends in gastro-enteritis in the Netherlands. Lowest incidences in 2002 ever; the lull before the storm? [in Dutch] Infectieziekten Bull. 2003;12:424–430. [Google Scholar]
- 12.Threlfall EJ, Fisher IST, Berghold C et al. Antimicrobial drug resistance in isolates of Salmonella enterica from cases of salmonellosis in humans in Europe in 2000: results of international multi-centre surveillance. Euro Surveill. 2003;8:41–45. doi: 10.2807/esm.08.02.00400-en. [DOI] [PubMed] [Google Scholar]
- 13.Rabatsky-Ehr T, Whichard J, Rossiter S et al. Multidrug-resistant strains of Salmonella enterica Typhimurium, United States, 1997–1998. Emerg Infect Dis. 2004;10:795–801. doi: 10.3201/eid1005.030209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Glynn MK, Bopp C, Dewitt W, Dabney P, Mokhtar M, Angulo FJ. Emergence of multidrug-resistant Salmonella enterica serotype Typhimurium DT104 infections in the United States. N Eng J Med. 1998;338:1333–1338. doi: 10.1056/NEJM199805073381901. [DOI] [PubMed] [Google Scholar]
- 15.Kimura AC, Reddy V, Marcus R et al. Chicken consumption is a newly identified risk factor for sporadic Salmonella enterica serotype Enteritidis infections in the United States: a case-control study in FoodNet sites. Clin Infect Dis. 2004;38:S244–S252. doi: 10.1086/381576. [DOI] [PubMed] [Google Scholar]
- 16.Hedberg CW, David MJ, White KE, MacDonald KL, Osterholm MT. Role of egg consumption in sporadic Salmonella Enteritidis and Salmonella Typhimurium infections in Minnesota. J Infect Dis. 1993;167:107–111. doi: 10.1093/infdis/167.1.107. [DOI] [PubMed] [Google Scholar]
- 17.Schmid H, Burnens AP, Baumgartner A, Oberreich J. Risk factors for sporadic salmonellosis in Switzerland. Eur J Clin Microbiol Infect Dis. 1996;15:725–732. doi: 10.1007/BF01691959. [DOI] [PubMed] [Google Scholar]
- 18.M⊘lbak K, Neimann J. Risk factors for sporadic infection with Salmonella Enteritidis, Denmark, 1997–1999. Am J Epidemiol. 2002;156:654–661. doi: 10.1093/aje/kwf096. [DOI] [PubMed] [Google Scholar]
- 19.Hayes S, Nylen G, Smith R, Salmon RL, Palmer SR. Undercooked hens eggs remain a risk factor for sporadic Salmonella Enteritidis infection. Commun Dis Public Health. 1999;2:66–67. [PubMed] [Google Scholar]
- 20.Cogan TA, Humphrey TJ. The rise and fall of Salmonella Enteritidis in the UK. J Appl Microbiol. 2003;94:114S–119S. doi: 10.1046/j.1365-2672.94.s1.13.x. [DOI] [PubMed] [Google Scholar]
- 21.Banatvala N, Cramp A, Jones IR, Feldman RA. Salmonellosis in North Thames (East), UK: associated risk factors. Epidemiol Infect. 1999;122:201–207. doi: 10.1017/s0950268899002162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cody SH, Abbott SL, Marfin AA et al. Two outbreaks of multidrug-resistant Salmonella serotype Typhimurium DT104 infections linked to raw-milk cheese in Northern California. J Am Med Assoc. 1999;281:1805–1810. doi: 10.1001/jama.281.19.1805. [DOI] [PubMed] [Google Scholar]
- 23.Delarocque-Astagneau E, Bouillant C, Vaillant V, Bouvet P, Grimont P, Desenclos J. Risk factors for the occurrence of sporadic Salmonella enterica serotype Typhimurium infections in children in France: a national case-control study. Clin Infect Dis. 2000;31:488–492. doi: 10.1086/313990. [DOI] [PubMed] [Google Scholar]
- 24.Davies A, O’Neill P, Towers L, Cooke M. An outbreak of Salmonella Typhimurium DT104 food poisoning associated with eating beef. Comm Dis Rep. 1996;6:159–162. [PubMed] [Google Scholar]
- 25.Delpech V, McAnulty J, Morgan K. A salmonellosis outbreak linked to internally contaminated pork meat. Aust NZ J Public Health. 1998;22:243–246. doi: 10.1111/j.1467-842x.1998.tb01181.x. [DOI] [PubMed] [Google Scholar]
- 26.Doré K, Buxton J, Henry B et al. Risk factors for Salmonella Typhimurium DT104 and non-DT104 infection: a Canadian multi-provincial case-control study. Epidemiol Infect. 2004;132:485–493. doi: 10.1017/s0950268803001924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Anon. Defective pasteurization linked to outbreak of Salmonella Typhimurium definitive phage type 104 infection in Lancashire. Commun Dis Rep CDR Wkly. 1998;8:335–338. [PubMed] [Google Scholar]
- 28.Kapperud G, Lassen J, Hasseltvedt V. Salmonella infections in Norway: descriptive epidemiology and a case-control study. Epidemiol Infect. 1998;121:569–577. doi: 10.1017/s0950268898001551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Duijkeren E van, Wannet WJB, Houwers DJ, Pelt W van. Antimicrobial susceptibilities of Salmonella strains isolated from humans, cattle, pigs, and chickens in the Netherlands from 1984 to 2001. J Clin Microbiol. 2003;41:3574–3578. doi: 10.1128/JCM.41.8.3574-3578.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Duijkeren E van, Wannet WJB, Houwers DJ, Pelt W van. Serotype and phage type distribution of Salmonella strains isolated from humans, cattle, pigs and chickens in the Netherlands from 1984 to 2001. J Clin Microbiol. 2002;40:3980–3985. doi: 10.1128/JCM.40.11.3980-3985.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rubin DB. Multiple imputation for nonresponse in surveys. New York: Wiley; 1987. [Google Scholar]
- 32.Greenland S, Finkle WD. A critical look at methods for handling missing covariates in epidemiologic regression analyses. Am J Epidemiol. 1995;142:1255–1264. doi: 10.1093/oxfordjournals.aje.a117592. [DOI] [PubMed] [Google Scholar]
- 33.Buuren S van, Oudshoorn CGM. 2000. Multivariate imputation by chained equations. MICE version 1.0 user’s manual. TNO report PG/GZ/00.038. Leiden: TNO Prevention and Health.
- 34.Schutze GE, Sikes JD, Stefanova R, Cave MD. The home environment and salmonellosis in children. Pediatrics. 1999;103:E1. doi: 10.1542/peds.103.1.e1. [DOI] [PubMed] [Google Scholar]
- 35.Santamaria J, Toranzos GA. Enteric pathogens and soil: a short review. Int Microbiol. 2003;6:5–9. doi: 10.1007/s10123-003-0096-1. [DOI] [PubMed] [Google Scholar]
- 36.Anderson GL, Caldwell KN, Beuchat LR, Williams PL. Interaction of a free-living soil nematode, Caenorhabditis elegans, with surrogates of foodborne pathogenic bacteria. J Food Prot. 2003;66:1543–1549. doi: 10.4315/0362-028x-66.9.1543. [DOI] [PubMed] [Google Scholar]
- 37.Glynn MK, Reddy V, Hutwagner L. Prior antimicrobial agent use increases the risk of sporadic infections with multidrug-resistant Salmonella enterica serotype Typhimurium: a FoodNet case-control study, 1996–1997. Clin Infect Dis. 2004;38:S227–S236. doi: 10.1086/381591. [DOI] [PubMed] [Google Scholar]
- 38.Neal KR, Briji SO, Slack RC, Hawkey CJ, Logan RF. Recent treatment with H2 antagonists and antibiotics and gastric surgery as risk factors for Salmonella infection. BMJ. 1994;308:176. doi: 10.1136/bmj.308.6922.176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Swift L, Hunter PR. What do negative associations between potential risk factors and illness in analytical epidemiological studies of infectious disease really mean. Eur J Epidemiol. 2004;19:219–223. doi: 10.1023/b:ejep.0000020453.84296.f6. [DOI] [PubMed] [Google Scholar]
- 40.Tam CC. Campylobacter reporting at its peak year of 1998: don’t count your chickens yet. Commun Dis Public Health. 2001;4:194–199. [PubMed] [Google Scholar]


