SUMMARY
Pulsed-field gel electrophoresis (PFGE) is commonly used in molecular epidemiology. However, this technique has never been used in studying intra-family spread of enteric diseases in Bangladesh. Our objective was to evaluate the intra-familial transmission of shigella infection using PFGE. Children of either sex, less than 10 years old, who were family contacts of shigella-infected index cases were the study population. PFGE was applied if the same serotypes/sub-serotypes of shigella were isolated from both the index case and the family contact children. In total, 227 index cases were studied. Shigella was isolated from 61 (27%) contact children on day 1 of enrolment, among which Shigella flexneri (41%) and S. boydii (41%) were dominant, followed by S. dysenteriae (10%), S. sonnei (3%), and shigella-like organisms (5%). Seventeen (28%) of the asymptomatic infections in contact children were caused by the same serotype of shigella as that found in the index case. The intra-familial shigella transmission rate was 8% (17/227). Of the 227 contact children, eight (4%) developed diarrhoea during a 10-day follow-up and shigella was isolated from five (2%) of these children, and three of them (S. flexneri 3a, 1b, and 3a) were identical to the strains from their respective index cases. Compared to children without asymptomatic carriage of shigella (2/166), the risk (odds ratio) of developing diarrhoea for the children with asymptomatic carriage of shigella identical to their cases (3/17) was 9·0 (95% CI 1·5–49·0, P=0·01). The attributable risk for symptomatic shigella infection by intra-familial transmission was 50%. Results of this study demonstrated that intra-familial transmission of shigella carries a higher risk for diarrhoea.
INTRODUCTION
Shigellosis is common among children in developing countries with poor hygiene and unsafe water supplies [1–3]. Transmission usually occurs through contaminated food and water, or through person-to-person contact [4, 5]. Ingestion of as few as 10 organisms can cause an infection, particularly in ‘toddlers’ who are not fully toilet-trained [6]. Infections are more severe in malnourished children who are also at higher risk of death [7]. Family members and playmates of such children are at an increased risk for acquiring the disease [4].
Strain discrimination is important in epidemiological studies. Analyses of plasmid profiles and antimicrobial susceptibility patterns have been used in the past to discriminate strains of Shigella spp. However, these methods have limitations as plasmids are unstable and the antimicrobial resistance profile, sometimes encoded on mobile elements, changes over a short period of time [8]. A variety of different typing methods have been used for strain discrimination, including pulsed-field gel electrophoresis (PFGE), ribotyping, rapid amplification of polymorphic DNA (RAPD), plasmid profiling, and PCR. PFGE has been successfully employed for strain discrimination of a variety of bacteria including Shigella spp. [9–12], and has become a useful tool in the study of molecular epidemiology. Several investigators have shown that strain discrimination by PFGE is superior to other methods [9]. The aim of this study was to determine the extent of intra-familial transmission of shigella in an endemic setting, using the powerful technique of PFGE and to compare this with conventional serotyping methods.
MATERIALS AND METHODS
Study design
The study was conducted at the Dhaka Hospital of ICDDR,B: Centre for Health and Population Research from January 2000 to September 2001. Initially patients of all age groups and of both sexes attending the hospital with a history of mucoid/bloody diarrhoea of less than 96 h duration, with a child aged <10 years in the family, were identified. Those patients with more than 10 leukocytes and at least one erythrocyte per high power field in stool microscopy of their freshly passed stool were designated as presumptive cases of shigellosis and stool was sent for culture. Patients whose stool culture yielded a shigella isolate were then enrolled as an index case.
After obtaining written informed consent from the patient or their legal guardian, the homes of the index cases were visited by a trained research assistant within 12–24 h of the patient reporting to hospital, and data was collected from a reliable family member using standard forms. Children of the same family, aged 12–120 months, and of either sex were, with parental consent, enrolled as contacts. If a family had more than one eligible contact then only the youngest child, being more vulnerable to disease, was enrolled. The contact children were enrolled if they were present in the household on the first day of the study and were free from diarrhoea. A rectal specimen was collected from these contacts on the day of enrolment, and subsequently if and when diarrhoea developed; research assistants visited study households on each of the next 10 days. A secondary case was defined as a family contact child who subsequently developed diarrhoea and yielded the same serotype and genotype of shigella as the index case.
Children with diarrhoea on the day that the suspected index case was identified, those with features of systemic infections or conditions requiring antibiotic therapy, severe malnutrition requiring immediate hospitalization, congenital anomalies, not residents of the community, and whose parents/guardians did not agree to participation were excluded. The study was approved by the Research Review Committee and the Ethical Review Committee of ICDDR,B.
Anthropometric measurements (weight, and length/height) of study children were recorded to determine their nutrition status. Weights were calculated to the nearest 100 g using an electric digital scale (Seca model 770; Seca, Hamburg, Germany) calibrated using a 1-kg standard weight on each day. Length/height was determined with a locally constructed length board on which a model, non-expandable tape was fixed between the footplate and head bar, on one side of the board. The mean of two consecutive measurements to the nearest 0·1 cm was recorded and considered as the actual value. All measurements were subsequently compared to the standards according to the National Centre for Health Statistics data and the nutritional status was assessed by Z scores.
Laboratory diagnosis
Freshly collected stool specimens were inoculated onto MacConkey and Salmonella–Shigella agar plates (Difco, Becton Dickinson & Company, Sparks, MD, USA). Shigella were isolated and identified using standard microbiological and biochemical methods [13]. Serotyping was performed using commercial antisera kits (Denka Seiken, Tokyo, Japan) specific for polyvalent and monovalent antigens for all serovars of shigella [14]. For serotyping of S. flexneri, a panel of monoclonal antibodies specific for all S. flexneri type and group-factor antigens (Reagensia AB, Stockholm, Sweden) was used. Strains were subcultured on MacConkey agar plates, incubated for ∼18 h, and serotyping was performed by slide agglutination test as previously described [15].
PFGE of genomic DNA from clinical isolates of shigella was performed as previously described [11, 16, 17], but with different pulse times: 3–28 s for 8 h, 5–50 s for 8 h, 20–80 s for 11 h, and 60–120 s for 11 h. Genomic DNA was digested with the NotI restriction enzyme (Gibco-BRL, Gaithersburg, MD, USA), and the restriction fragments were separated using CHEF-DRII system in 1% pulsed-field certified agarose in 0·5× TBE buffer. The gel was stained, de-stained, and photographed on a gel documentation system according to procedures previously described [14]. The DNA size standards used were: the bacteriophage lambda ladder ranging from 48·5 to 1000 kb (Bio-Rad), and Saccharomyces cerevisiae chromosomal DNA ranging from 225 to 2200 kb (Bio-Rad). Band patterns were established as previously described [9].
Bacteria isolated both from the index cases and study children were confirmed as Shigella spp. at the serotype and sub-serotype level using conventional methods and further PFGE analysis was applied if the same types were isolated from the index case and contact.
Statistical methods
Data were entered and statistical analyses were performed using SPSS PC+ for Windows (SPSS Inc., Chicago, IL, USA) and Epi-Info (CDC, Atlanta, GA, USA, and WHO). The significance of difference in proportions was evaluated by χ2 test and Fisher’s exact test was applied when appropriate. A probability of <0·05 was considered statistically significant.
RESULTS
Stool specimens of a total of 561 individuals were screened, of whom 389 patients with a presumptive diagnosis of shigellosis were initially enrolled into the study and 172 were excluded, as microscopic examination of 171 patients did not suggest a presumptive diagnosis of shigellosis, and the other patient declined to participate. Of the remaining 389 patients, 162 were excluded due to failure to isolate shigella from their stool. Finally, 227 (58%) patients with culture-proven shigella infections were retained as index cases of whom 133 (59%) were males. Seventeen per cent of the index cases were 0–12 months old, and the highest prevalence (24%) was among children aged 13–24 months (Table 1). Distribution of the shigella types is shown in Table 2. S. flexneri was the most common (n=122, 54%), followed by S. dysenteriae (n=45, 20%), S. boydii (n=38, 16%), and S. sonnei (n=22, 10%).
Table 1.
Age distribution of index cases and contact children
Table 2.
Distribution of Shigella spp. and their serotypes and sub-serotypes

Family contact/study children
The mean±s.d. age of the study children was 94±19 months, with 53% male, and only nine (4%) <5 years old. Stunting and underweight were the most common types of malnutrition of the contact children, 8% were severely stunted and 4% were severely underweight. Over 83% of children had completed immunizations according to Expanded Programme on Immunization (EPI) schedules. The mean±s.d. age of mothers of the study children was 31±6 years (data not shown).
Asymptomatic infections in the study children
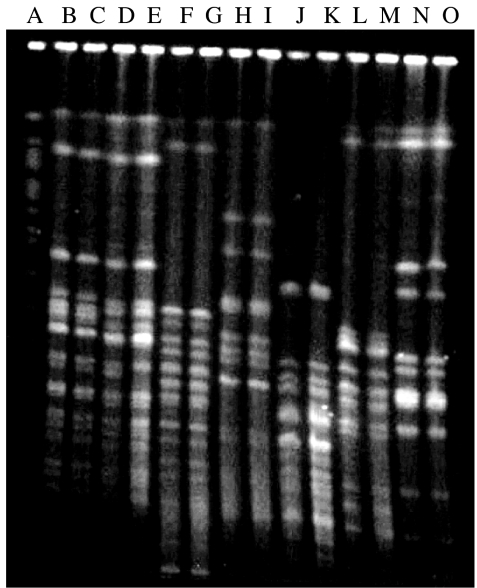
Rectal swab cultures of 61 (27%) children yielded shigella of different serotypes and sub-serotypes on day 1 of enrolment, which comprised of S. flexneri (41%), S. boydii (41%), S. dysenteriae (10%), S. sonnei (3%), and 5% shigella-like organisms (strains which had characteristics typical of shigella but could not be identified and were, therefore, designated as shigella-like organisms) (Table 3). Of the 25 isolates of S. flexneri, S. flexneri type 1 was most common (n=7), followed by S. flexneri 2a (n=4), S. flexneri 2b (n=3), and S. flexneri 3a (n=2) (Table 3). Children with or without shigella isolated from their rectal swab did not significantly differ in their socio-economic-demographic characteristics (data not shown). The same serotypes of Shigella spp. were isolated from 28% (17/61) of the asymptomatic infections among contact children of the index cases (Table 4). This finding was based on the identical banding patterns observed in PFGE analysis (Fig.). The intra-familial transmission rate of asymptomatic shigella infection in the study population was 8% on day 1 (17/227).
Table 3.
Distribution of serotypes and sub-serotypes of Shigella spp. During asymptomatic infection of contact children on day 1 of enrolment

Table 4.
Age and sex distribution of identical shigella among index cases and contact children
Fig.
PFGE patterns of NotI-digested chromosomal DNA from representative strains of Shigella spp. isolated from index cases (IC) and study children (SC). Lanes: A, Saccharomyces cerevisiae (marker); B, 4085, Shigella flexneri 2b (IC); C, 4093, S. flexneri 2b (SC); D, 5061, S. flexneri 2a (IC); E, 5082, S. flexneri 2a (SC); F, 25953, S. flexneri type 6 (IC), G, 26028, S. flexneri type 6 (SC); H, 24744, S. dysenteriae 2 (IC); I, 24797, S. dysenteriae 2 (SC); J, 13096, S. boydii 8 (IC); K, 13110, S. boydii 8 (SC); L, 8172, S. boydii 11 (IC); M, 8173, S. boydii 11 (SC); N, 6668, S. sonnei (IC); O, 6740, S. sonnei (SC).
Carriage of identical shigella has a greater risk of diarrhoea
Of the 227 contact children, eight (4%) developed diarrhoeal disease during the 10-day follow-up period, most of these episodes developed during the later part of follow-up, and shigella was isolated from rectal swab of only five (2%) of them (Table 5). Of these five Shigella spp., identical serotypes of shigella (S. flexneri 3a, 1b, and 3a) were identified from three children. These three contact children who developed diarrhoea and had identical isolates were in the 17 out of 61 contacts of index cases. PFGE analysis of NotI-digested chromosomal DNA of the shigella isolated from index cases and contact children yielded 20–23 reproducible DNA fragments ranging in size from approximately 15 to 750 kb. The banding pattern of the shigella isolated from index cases was identical to the banding pattern of the same strain isolated from a study child (Fig.). Two out of five study children developed diarrhoea during their follow-up and shigella were isolated from their rectal specimen, which were different from their index cases. One child with asymptomatic infection on the first day developed diarrhoea during follow-up, however, no pathogen (Vibrio cholerae, salmonella, shigella) could be isolated from his stool. Two out of 166 (1%) with negative rectal swab culture for shigella on the first day also developed diarrhoea, but none had a rectal swab culture positive for any of the above pathogens. Compared to children without asymptomatic carriage of shigella (2/166), the risk (odds ratio) of developing diarrhoea for the children with asymptomatic carriage of shigella identical to their cases (3/17) was 9·0 (95% CI 1·5–49·0, P=0·01). The attributable risk for shigella-associated diarrhoea due to intra-familial transmission was 50%.
Table 5.
Shigella serotypes isolated from the contact children during follow-up
The PFGE patterns of the shigella serotypes of the index cases and contact children were identical.
DISCUSSION
Personal contact has been documented as being responsible not only for small-scale transmission among household contacts and communities, but also for the spread over a much wider geographical area [18]. PFGE has been found to be a very useful tool for laboratory-based surveillance for public health purposes. Wider application of this method may add to a better understanding of molecular epidemiology of community-acquired infections, outbreak investigations, and mode of transmission. Therefore, PFGE allows application of better control and preventive measures.
The present study, the first in Bangladesh, applied two epidemiological typing methods (conventional and molecular techniques) to confirm and delineate the patterns of transmission of the shigella infections in an urban setting. Similar studies need to be conducted in rural areas of Bangladesh [19, 20]. The same serotypes of Shigella spp. were isolated from 28% (17/61) of the asymptomatic infections among contact children of the index cases. The intra-familial transmission rate of asymptomatic shigella infection in the study population was 8% on day 1 (17/227). The overall rate of asymptomatic infections was higher than reported earlier from rural Bangladesh, which could be related to the more densely living conditions in urban areas. Some limitations of the study warrant attention. The definition of asymptomatic carriage of shigella was based on only one stool specimen, rather than specimens of 10 consecutive days. Therefore, a contact who became infected but remained asymptomatic during the entire follow-up period was not detected by the study. Infants were less affected, similar observations indicating this have been reported earlier [21, 22]. Lower asymptomatic carriage in this age group may be related to breast-feeding since most of the children in Bangladesh are breast-fed during infancy [23, 24]. Similar distribution of S. boydii and S. flexneri among asymptomatic carriers indicates their equal importance, contrary to the common belief that S. flexneri is the most important.
The basic premise for the typing of isolates for epidemiological investigation is to understand and track the mode of transmission of infectious agents in order to define control measures, and different epidemiological tools are used for this purpose. In our study, we applied conventional serotyping and sub-serotyping methods for typing Shigella spp. isolated from index cases and their family contact children [25] and also applied the molecular method of PFGE for comparison when the same serotypes/sub-serotypes were isolated from both index cases and their family contact children. The PFGE patterns of the same serotype/sub-serotype isolated from index cases and study children were identical. The findings suggested that the sources of the infection of both the index cases and study children were the same.
Results of our study demonstrated that intra-familial transmission of shigella carries a risk of diarrhoea, nine-fold higher among children with intra-familial transmission than among children of households without intra-familial transmission. We estimate that prevention of intra-familial transmission of this organism could help reduce shigella-associated diarrhoeal morbidity by 50%.
ACKNOWLEDGEMENTS
This research protocol was funded by ICDDR,B: Centre for Health and Population Research with the support of the Swiss Agency for Development and Cooperation (SDC). ICDDR,B acknowledges with gratitude the commitment of SDC to the Centre’s research efforts. We thank Dr M. A. Salam, Dr Rubhana Raqib, and Dr S. M. Faruque for their thoughtful comments.
DECLARATION OF INTEREST
None.
REFERENCES
- 1.Hollister AC, Beck MD, Gittelsohn AM, Hemphill EC. Influence of water availability on Shigella prevalence in children of farm labor families. Am J Public Health. 1955;45:354–362. doi: 10.2105/ajph.45.3.354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bhat P, Myers RM, Feldman RA. Significance of the incidence of shigella infection as indicated by results of a longitudinal study. Jpn J Med Sci Biol. 1970;23:237–242. doi: 10.7883/yoken1952.23.237. [DOI] [PubMed] [Google Scholar]
- 3.Gordon JE, Behar M, Scrimshaw NS. Acute diarrhoeal disease in less developed countries. I. An epidemiological basis for control. Bull World Health Organ. 1964;31:1–7. [PMC free article] [PubMed] [Google Scholar]
- 4.el Bushra HE, Bin Saeed AA. Intrafamilial person-to-person spread of bacillary dysentery due to Shigella dysenteriae in southwestern Saudi Arabia. East Afr Med J. 1999;76:255–259. [PubMed] [Google Scholar]
- 5.Mosley WH, Adams B, Lyman ED. Epidemiologic and sociologic features of a large urban outbreak of shigellosis. J Am Med Assoc. 1962;182:1307–1311. doi: 10.1001/jama.1962.03050520005002. [DOI] [PubMed] [Google Scholar]
- 6.Shears P. Shigella infections. Ann Trop Med Parasitol. 1996;90:105–114. doi: 10.1080/00034983.1996.11813034. [DOI] [PubMed] [Google Scholar]
- 7.Ahmed F, Clemens JD, Rao MR, Ansaruzzaman M, Haque E. Epidemiology of shigellosis among children exposed to cases of shigella dysentery: a multivariate assessment. Am J Trop Med Hyg. 1997;56:258–264. doi: 10.4269/ajtmh.1997.56.258. [DOI] [PubMed] [Google Scholar]
- 8.Munshi MH, Sack DA, Haider K, Ahmed ZU, Rahman MM, Morshed MG. Plasmid-mediated resistance to nalidixic acid in Shigella dysenteriae type 1. Lancet. 1987;2:419–421. doi: 10.1016/s0140-6736(87)90957-3. [DOI] [PubMed] [Google Scholar]
- 9.Tenover FC, Arbeit RD, Goering RV et al. Interpreting chromosomal DNA restriction patterns produced by pulsed-field gel electrophoresis: criteria for bacterial strain typing. J Clin Microbiol. 1995;33:2233–2239. doi: 10.1128/jcm.33.9.2233-2239.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yagupsky P, Loeffelholz M, Bell K, Menegus MA. Use of multiple markers for investigation of an epidemic of Shigella sonnei infections in Monroe County, New York. J Clin Microbiol. 1991;29:2850–2855. doi: 10.1128/jcm.29.12.2850-2855.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Talukder KA, Dutta DK, Albert MJ. Evaluation of a pulsed-field gel electrophoresis for typing of Shigella dysenteriae type 1. J Med Microbiol. 1999;48:781–784. doi: 10.1099/00222615-48-8-781. [DOI] [PubMed] [Google Scholar]
- 12.Fernandez-Prada CM, Venkatesan MM, Franco AA et al. Molecular epidemiology of Shigella flexneri in a diarrhoea-endemic area of Lima, Peru. Epidemiol Infect. 2004;132:303–316. doi: 10.1017/s0950268803001560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.World Health Organization. Manual for laboratory investigations of acute enteric infections. Geneva: WHO; 1987. Programme for Control of Diarrhoeal Diseases (CDD/83.3 Rev 1) [Google Scholar]
- 14.Talukder KA, Islam MA, Dutta DK et al. Phenotypic and genotypic characterization of serologically atypical strains of Shigella flexneri type 4 isolated in Dhaka, Bangladesh. J Clin Microbiol. 2002;40:2490–2497. doi: 10.1128/JCM.40.7.2490-2497.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Talukder KA, Dutta DK, Safa A et al. Altering trends in the dominance of Shigella flexneri serotypes and emergence of serologically atypical S. flexneri strains in Dhaka, Bangladesh. J Clin Microbiol. 2001;39:3757–3759. doi: 10.1128/JCM.39.10.3757-3759.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Smith CL, Davies KE. Genome analysis: a practical approach. Washington, DC: IRL Press; 1988. Pulsed-field gel electrophoresis and the technology of large DNA molecules; pp. 41–72. [Google Scholar]
- 17.Albert MJ, Bhuiyan NA, Talukder KA et al. Phenotypic and genotypic changes in Vibrio cholerae 0139 Bengal. J Clin Microbiol. 1997;33:3119–3123. doi: 10.1128/jcm.35.10.2588-2592.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chiou CS, Hsu WB, Wei HL, Chen JH. Molecular epidemiology of a Shigella flexneri outbreak in a mountainous township in Taiwan, Republic of China. J Clin Microbiol. 2001;39:1048–1056. doi: 10.1128/JCM.39.3.1048-1056.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hossain MA, Hasan KZ, Albert MJ. Shigella carriers among non-diarrhoeal children in an endemic area of shigellosis in Bangladesh. Trop Geogr Med. 1994;46:40–42. [PubMed] [Google Scholar]
- 20.Ahmed F, Clemens JD, Rao MR, Banik AK. Family latrines and paediatric shigellosis in rural Bangladesh: benefit or risk. Int J Epidemiol. 1994;23:856–862. doi: 10.1093/ije/23.4.856. [DOI] [PubMed] [Google Scholar]
- 21.Guerrero L, Calva JJ, Morrow AL et al. Asymptomatic Shigella infections in a cohort of Mexican children younger than two years of age. Pediatr Infect Dis J. 1994;13:597–602. doi: 10.1097/00006454-199407000-00003. [DOI] [PubMed] [Google Scholar]
- 22.Clemens JD, Stanton B, Stoll B, Shahid NS, Banu H, Chowdhury AK. Breast feeding as a determinant of severity in shigellosis. Evidence for protection throughout the first three years of life in Bangladeshi children. Am J Epidemiol. 1986;123:710–720. doi: 10.1093/oxfordjournals.aje.a114291. [DOI] [PubMed] [Google Scholar]
- 23.Glass RI, Stoll BJ. The protective effect of human milk against diarrhea. A review of studies from Bangladesh. Acta Paediatr Scand. 1989;351:131–136. doi: 10.1111/j.1651-2227.1989.tb11225.x. [DOI] [PubMed] [Google Scholar]
- 24.Ahmed F, Clemens JD, Rao MR, Sack DA, Khan MR, Haque E. Community-based evaluation of the effect of breast-feeding on the risk of microbiologically confirmed or clinically presumptive shigellosis in Bangladeshi children. Pediatrics. 1992;90:406–411. [PubMed] [Google Scholar]
- 25.Khan AI, Huq S, Malek MA et al. Shigella serotypes among hospitalized patients in urban Bangladesh and their antimicrobial resistance. Epidemiol Infect. 2004;132:773–777. doi: 10.1017/s0950268804002134. [DOI] [PMC free article] [PubMed] [Google Scholar]