SUMMARY
In May 2000, public health authorities in Dublin, Ireland, identified a cluster of unexplained severe illness among injecting drug users (IDUs). Similar clusters were also reported in Scotland and England. Concurrent investigations were undertaken to identify the aetiology and source of the illnesses. In Dublin, 22 IDUs were identified with injection-site inflammation resulting in hospitalization or death; eight (36%) died. Common clinical findings among patients with severe systemic symptoms included leukaemoid reaction and cardiogenic shock. Seventeen (77%) patients reported injecting heroin intramuscularly in the 2 weeks before illness. Of 11 patients with adequate specimens available for testing, two (18%) were positive by 16S rDNA PCR for Clostridium novyi. Clinical and laboratory findings suggested that histotoxic Clostridia caused a subset of infections in these related clusters. Empiric treatment for infections among IDUs was optimized for anaerobic organisms, and outreach led to increased enrolment in methadone treatment in Dublin. Many unique legal, medical, and public health challenges were encountered during the investigation of this outbreak.
INTRODUCTION
On 19 May 2000, public health authorities in the United Kingdom reported a cluster of severe unexplained illnesses among injecting drug users (IDUs) in Glasgow, Scotland [1]. On 20 May 2000, emergency department physicians from a hospital in southwest Dublin, Ireland notified the coroner of an unexplained death with temporal and social links to a cluster of severe illnesses among IDUs. By 25 May 2000, the Eastern Regional Health Authority (ERHA) in Ireland had identified a total of 14 similar cases among IDUs presenting to this hospital [2]. Shortly thereafter, a third cluster was identified among IDUs in northwest England [3]. In all locations, the initial case-patients shared distinct historical and clinical findings, including recent heroin use and severe soft-tissue inflammation at an injection site, often followed by profound leucocytosis and cardiogenic shock. Post-mortem examinations did not reveal a specific cause of death [4].
In mid-May Norwegian authorities reported that an IDU from Oslo had recently died from anthrax [5, 6]. The clinical presentation was very similar to the clusters identified in the United Kingdom and Ireland. Coupled with the expanding number of cases and severity of illness, this report heightened the sense of urgency to identify the cause of the outbreak. In response, a collaborative international investigation was initiated to (1) describe the clinical and epidemiological features of the case patients, (2) identify the cause of their unexplained illness, and (3) define a potential source of the outbreak. This paper describes the epidemiology of the Irish outbreak of severe illness and death in IDUs and highlights the unique challenges of this public health investigation.
METHODS
Case finding
For the purposes of this investigation, a case was defined as an IDU admitted to hospital or found dead in Ireland between 1 April and 31 August 2000 with severe soft-tissue inflammation (i.e. abscess, induration, cellulitis, fasciitis, myositis) at an injection site. Active and enhanced passive surveillance was conducted through inquiries to emergency departments, hospital pathologists, coroners’ offices and drug treatment centres in the ERHA. Public health alerts were also disseminated to all hospitals and general practitioners in Ireland and the United Kingdom.
Data collection
We reviewed available medical records and autopsy reports for each case-patient. Variables collected included patient demographics, underlying conditions, clinical signs and symptoms, laboratory findings, treatment, illness course, outcome, and post-mortem findings. Surviving patients were interviewed regarding clinical symptoms, living conditions and drug use practices including types and sources of drugs used, drug preparation, routes of injection, sharing of drugs or equipment, and recent changes in behaviour. For deceased patients, surrogates familiar with their routine habits and exposures were interviewed.
Laboratory procedures and testing
Gram stain and routine aerobic and anaerobic cultures were performed at local microbiology laboratories on available blood and wound samples. When possible, specimens were also sent to reference laboratories at the Centers for Disease Control and Prevention (CDC, Atlanta, GA, USA) and the Public Health Laboratory Service (PHLS, UK) for further diagnostic testing.
Tissue samples were evaluated using enhanced aerobic and anaerobic culture, histopathology, immunohistochemistry (IHC) against group A streptococcus and Bacillus anthracis, and broad range bacterial 16S ribosomal DNA (16S rDNA) polymerase chain reaction (PCR). Enhanced aerobic and anaerobic cultures and isolate identification were performed based on a standard outbreak protocol [7]. Isolates identified as Clostridium spp. were subtyped using amplified fragment length polymorphism (AFLP) and screened for toxin production [8, 9]. Heroin samples, citric acid and equipment thought to be associated with the outbreak were evaluated by various aerobic and anaerobic culture methods [10].
Evaluation of an intervention strategy
During the outbreak, methadone maintenance treatment (MMT) was promoted as an alternative to using potentially contaminated heroin or related materials. Outreach activities focused primarily on southwest Dublin, the area most affected by the outbreak. To assess the effectiveness of this campaign, the number of IDUs from southwest Dublin that enrolled into MMT was determined for the 4 months after the outbreak was recognized (i.e. June–September 2000) and compared to data from the same period of time 1 year prior (i.e. June–September 1999). To determine treatment retention rates at 1 year for persons enrolled during the outbreak, registration data was also evaluated for June–September 2001.
Data analysis
Data were collected on a standardized form and analysed using Epi-Info 2000 (CDC). Categorical variables were described as proportions and compared using the Mantel–Haenszel χ2 or Fisher’s exact test. Continuous variables were described by the median and range and compared using the Kruskal–Wallis Wilcoxon rank sum test.
RESULTS
Patient demographics
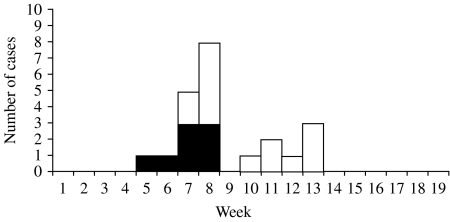
Between 1 April and 31 August 2000, we identified 22 IDUs in Ireland with severe injection-site inflammation resulting in hospitalization or death; eight (36%) cases were fatal. The first patient was admitted to hospital on 2 May and the last presented on 26 June (Fig. 1). The case-fatality ratio varied over the course of the outbreak, dropping from 53% (8/15) for IDUs first hospitalized in May compared to 0% (0/7) for patients presenting in June (P=0·02). The median age of case-patients was 30 years (range 19–51 years); 14 (64%) were male. In Ireland, all identified case-patients resided in the Dublin metropolitan area, including 14 (64%) who lived in a single small neighbourhood in southwest Dublin. No cases were identified from several other areas in the city with large populations of known IDUs.
Fig. 1.
Cases of severe illness among injecting drug users by week of hospitalization, Ireland, 1 April to 31 August 2000. □, Non-fatal; ■, fatal.
Clinical characteristics
Many of the clinical observations among the Irish case-patients were similar to those seen among Scottish and English cases [11, 12]. The chief presenting complaint among most patients was soft-tissue infection of an injection site located on the buttocks (32%), groin (18%), thigh (18%), arm (14%), abdomen (9%), or an in-dwelling catheter site (5%). These wound infections were characterized by oedema (86%), erythema (73%), pain (68%), necrosis (23%), pus (14%) and crepitus (5%). Six (27%) patients had accompanying lymphadenopathy noted. Among the 21 case-patients for whom data were available, there was a median of 5 days (range 0–14 days) between symptom onset and hospitalization. Systemic signs and symptoms at hospitalization included fever 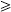 38·0°C (50%), altered mental status (32%), extreme thirst (23%), hypotension (18%), and nausea/vomiting (18%).
38·0°C (50%), altered mental status (32%), extreme thirst (23%), hypotension (18%), and nausea/vomiting (18%).
Several important clinical features were not prominent findings among these patients. Few patients had a rash outside of the wound site. Thrombocytopenia, disseminated intravascular coagulation, liver failure and renal failure were also uncommon. Although a number of patients developed altered mental status, none had focal neurological changes indicative of botulism or tetanus. Finally, only two (9%) patients were known to be infected with human immunodeficiency virus (HIV), including one fatal and one non-fatal case; 10 (45%) patients were known to be infected with hepatitis C virus, and five (23%) patients were known to be infected with hepatitis B virus.
Although systemic symptoms were not prominent features at presentation, more than one third of the patients experienced a subsequent rapid deterioration including hypotension and cardiogenic shock, often despite surgical debridement and broad-spectrum antibiotics. Among the eight fatal cases, there was a median of only 1 day (range 0–7 days) from admission to death. Altered mental status (i.e. agitation or confusion) was noted in five (63%) of eight fatal cases, compared with only two (14%) of the 14 non-fatal patients (Fisher’s exact P=0·05). The median WBC among fatal cases was 60 000 cells/mm3 (range 8200–96 500 cells/mm3) compared with 11 950 cells/mm3 (range 2800–17 300 cells/mm3) for survivors (Kruskal–Wallis P=0·02). One patient who subsequently died had a WBC of 13 500 cells/mm3 at presentation and 81 700 cells/mm3 less than 24 h later, a pattern consistent with a leukaemoid reaction. HIV status, severity of wound site infection (e.g. necrosis or crepitus) and fever were not predictive of poor outcome.
Of the eight case-patients who died, post-mortem examination of the injection-site wounds (i.e. skin, subcutaneous tissue, fascia and muscle) revealed swelling or oedema (75%) and pus or abscess formation (25%). All had evidence of pulmonary oedema. Other common systemic autopsy findings included large pleural and pericardial effusions (88%), subendocardial haemorrhage (63%), and hepatosplenomegaly (63%). As presented by Finn et al. [13] toxicological evaluation revealed that all fatal cases had narcotics, including methadone and free morphine present at either toxic or fatal levels; however, based on the history of habitual drug use, clinical presentations, and autopsy findings, drug overdose was not considered the likely cause of death.
Patient and surrogate interviews
Nineteen patients or their surrogates were available for interview. Of these, 14 (74%) injected heroin alone and five (26%) injected heroin and cocaine together. All injected with needles provided by the needle exchange, and all used citric acid to dissolve the heroin prior to injecting. Twelve cases (63%) claimed the heroin to be different by colour, consistency, and/or sensation upon injecting, and nine (47%) described the heroin to be harder to dissolve and required more citric acid than normal. Seventeen cases (89%) injected drugs into skin or muscle (i.e. popping) because of lack of available vein access, and two (11%) had failed intravenous injection (i.e. missed the vein) at the site of their soft-tissue infection.
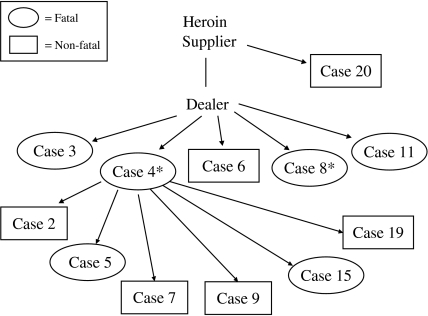
Twelve (55%) of the case-patients interviewed could be directly or indirectly linked to a common supplier of heroin (Fig. 2). Furthermore, six (43%) of the 14 non-fatal patients were family or close friends to those who died and had injected the same batch of heroin together. These patients claimed to have sought treatment earlier than they would have normally because of the dramatic decline and death of their injecting partner.
Fig. 2.
Potential links to a common supplier of heroin for 12 (55%) of the 22 cases identified in Ireland between 1 April and 31 August 2000. * C. novyi isolated from clinical specimens.
Laboratory findings
Specimens from the injection-site wound were available for diagnostic testing on 11 (50%) of the 22 patients identified, including five (63%) of eight fatal cases and six (43%) of 14 non-fatal cases. Based on results from all laboratory sites, nine (81%) of the 11 patients had one or more well-described aetiologies of soft-tissue infection among IDUs identified, including Clostridium novyi (n=2), Staphylococcus aureus (n=3) and S. aureus and β-haemolytic streptococcus (n=3) (Table). Two additional patients had abundant Gram-positive rods identified from the wound site, but tissue was not available for culture and a single organism could not be discerned by 16S rDNA PCR on fixed specimens. Further confounding the process of defining an aetiology, six (55%) of the 11 patients also had 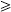 1 organism isolated that are less commonly associated with wound site infections, including Enterococcus spp. (n=1), peptostreptococcus (n=1), Escherichia coli (n=1), Candida albicans (n=1), and mixed flora (n=1). There was no evidence of Bacillus anthracis in any specimens tested.
1 organism isolated that are less commonly associated with wound site infections, including Enterococcus spp. (n=1), peptostreptococcus (n=1), Escherichia coli (n=1), Candida albicans (n=1), and mixed flora (n=1). There was no evidence of Bacillus anthracis in any specimens tested.
Table.
Microbiological findings from wound-site specimens among injecting drug users with severe illness or death, Ireland, April–August 2000
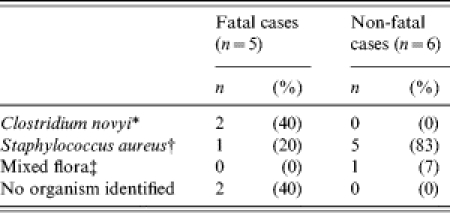
One patient had C. novyi isolated from the wound site as part of a mixed culture that included peptostreptococcus, enterococcus, Bacillus fragilis and Curvularia.
All six patients with evidence of infection due to S. aureus had another organism isolated from the wound site including group C streptococcus (2), group B streptococcus (1), α-haemolytic streptococcus (1), Enterococcus spp. (1) and Candida albicans (1).
One non-fatal case had mixed flora isolated from the wound site that included Clostridium argentensis, Clostridium subterminale, Enterococcus spp., and Curvularia.
Despite the variable and polymicrobial findings, there was evidence that two of the more severe infections were attributable to toxin-producing C. novyi. Both patients with evidence of C. novyi infection died, and both were epidemiologically linked to a common supplier of heroin. The one isolate available for testing showed evidence of toxin production by mouse and Vero cell biotoxin assays. This isolate was identified as type A by conventional and 16S rDNA sequencing and was identical by AFLP to 15 C. novyi type A isolates obtained from IDUs in the United Kingdom during the outbreak period [9]. By contrast, only one (17%) of the six patients with evidence of S. aureus or β-haemolytic streptococcus died.
Adequate heroin samples were available for testing from only two patients, neither of who had clinical specimens for diagnostic testing; Clostridium perfringens was isolated from one heroin sample and a Bacillus spp. from the other.
Intervention outcomes
At the beginning of the outbreak, approximately 5000 heroin users were registered with methadone treatment centres in Dublin. Following a concerted effort to promote MMT as an alternative to using potentially contaminated heroin or related materials, 136 IDUs from southwest Dublin were enrolled into MMT between June and September 2000. By contrast, 86 new users were enrolled from that neighbourhood during June–September 1999. After 1 year, 77% of the individuals who enrolled during the outbreak period in 2000 remained in treatment, which is comparable to the retention rate seen among IDUs who enrol in MMT for any reason [14].
DISCUSSION
Public health authorities faced several unique challenges in the investigation of this outbreak. Distinct characteristics of the IDU population created multiple obstacles including (1) the initial detection of the outbreak and the aetiological agent, (2) identifying risk factors for infection, and (3) designing an appropriate intervention strategy. IDUs have high rates of morbidity and mortality due to soft-tissue infection, other infectious diseases such as HIV and viral hepatitis, and overdose [15–17]. A review of Dublin coroners’ records identified that 84 opiate-related deaths occurred during 1999 [18]. At that time, Dublin had a population of 1·1 million with an estimated 13 000 IDUs [19]. While limited to 1 year of data, these results suggest that the high background of opiate-related deaths may have precluded identifying this cluster by a review of overall mortality among IDUs. As a result, detection of this outbreak was highly dependent on astute observations of clinicians and pathologists, in combination with the temporal association with similar clusters of severe illness and death among IDUs in the United Kingdom [1, 3, 4, 20].
Identification of an aetiological agent was hampered by several factors, including the high prevalence of soft-tissue infection in this population. Previous studies among IDUs have demonstrated a prevalence of soft-tissue infections or cellulitis of up to 32% [16, 21]. Given the already elevated rate of injection-site infections in this population, the use of a non-specific clinical case-definition may have resulted in the detection of sporadic cases that did not involve C. novyi infection. S. aureus and Streptococcus spp., both well-recognized causes of soft-tissue infection among IDUs, were isolated from 27% of Dublin case-patients. In addition, the presence of toxic and fatal levels of narcotics in all of the deaths in Ireland raises the question of a possible contribution of high drug levels to fatal outcome [13]. However, despite these confounding factors, 17% of case-patients in Ireland and the United Kingdom had evidence of infection with C. novyi type A, isolates of which were indistinguishable by AFLP [22]. This microbiological evidence, in combination with the distinct clinical findings, suggest that C. novyi was the causative agent of severe illness in at least a subset of case-patients.
Further complications were encountered in the process of the epidemiological investigation. Case-patients and surrogates were often concerned about disclosing information regarding their activities or the source of their heroin for fear of their safety and the possibility of criminal prosecution. However, during the interview process, we were able to link 12 of the 22 case-patients to a common supplier of heroin. Six of the 12 linked case-patients died, including the two fatal cases with evidence of C. novyi infection (cases 4 and 8). This traditional epidemiological approach can provide further evidence that a subset of case-patients shared a common source of potentially contaminated heroin. This type of approach or findings were not available for the investigations in Scotland and England [13, 14].
Risk behaviours, such as needle-sharing and skin-popping may be practised by many IDUs, leading to possible confounding in epidemiological analyses. Despite these challenges, evidence from a case-control study comparing Dublin case-patients to IDUs with no recent history of soft-tissue infection demonstrated that injection into skin or muscle was an independent risk factor for being a case-patient [23]. A case-control study of the Scottish cases identified injection into the skin or muscle and using a filter shared with someone else as variables strongly associated with illness [24]. Similar injecting behaviour was also evident among case-patients from England [11]. Injection into skin or muscle has been associated with outbreaks of other clostridial infections such as botulism and tetanus. In these outbreaks, investigators surmised that tissue damage resulting from skin-popping may have generated an anaerobic environment ideal for the development of clostridial infections [25–28].
Limitations in laboratory diagnosis and specimen collection were additional challenges to the public health investigation. Clostridia are highly fastidious, Gram-variable organisms that are difficult to grow and identify under standard diagnostic conditions. This may have interfered with identification at local microbiology laboratories. Specimens from the injection-site wound were only available for 50% of case-patients, precluding laboratory evaluation for a possible aetiology. Delays in autopsies on fatal cases, particularly early in the outbreak period may have resulted in significant post-mortem contamination, making the interpretation of culture and other laboratory results difficult.
In an effort to improve case-finding, management and laboratory diagnosis, a variety of interventions were implemented. Rapid initial clinical improvement was seen in case-patients who received high-dose therapy with antimicrobials with known effectiveness against anaerobes (e.g. clindamycin). Health alerts recommended that drug users with injection site wounds be evaluated and treated as early as possible. Recommended treatment included surgical debridement and culture of any injection-site infection, and the use of antibiotics with good anaerobic activity as part of empiric therapy. Microbiology laboratories optimized procedures for the isolation and identification of anaerobic bacteria. No additional cases were identified after 26 June 2000 and a significant decrease was seen in the case-fatality ratio among case-patients presenting in June compared to those presenting in May. These findings demonstrate that interventions may have had some beneficial effect. However, the depletion of contaminated heroin, and the effect of earlier hospital presentation by cases occurring later in the outbreak may have also contributed to the decline in case fatality over time.
MMT was widely promoted as a means to avoid heroin use since it was felt that harm reduction strategies such as skin cleansing or syringe exchange may not have been adequate interventions given the suspected contamination of heroin or related materials. While motivating behavioural change among IDUs is typically challenging, a significant increase in the number of new enrolees into MMT was seen following the outbreak period in 2000. This may have been due in part to an aggressive outreach effort, although reasons for enrolment were never ascertained. Whether or not outreach activities were effective among IDUs at highest risk (i.e. those reporting skin-popping) cannot be determined. Approximately 40–50% of IDUs who have enrolled in MMT in other settings have reported continued heroin use despite treatment [14], however, the proportion of Dublin MMT enrolees that completely abstained from heroin use during their enrolment is not known. In addition, five of the eight deaths (63%) had detectable levels of methadone on autopsy toxicological evaluation, suggesting that case-patients may still have been at risk for infection from injecting despite the concurrent use of methadone [13]. There has been a gradual but steady increase in the provision of MMT in Dublin from 1800 at the end 1996 to 6500 at the end 2003 (J. Barry, personal communication). Thus, it is difficult to interpret the increase in localized recruitment between 1999 and 2000. Likewise examination of trends in other markers such as drug deaths and prevalence estimates casts no light on reasons for the increased recruitment into MMT.
In Scotland, community intervention primarily involved promoting risk reduction [12] as opposed to enrolment in MMT. For example, users were encouraged to smoke heroin instead of injecting. If injecting heroin was unavoidable, users were advised not to inject into skin or muscle and to reduce the amount of acid utilized. This approach differed from the Irish effort to promote MMT enrolment, the impact of which we have attempted to measure. It is unknown whether one approach was more successful over the other with regard to reducing the risk of severe illness and infections related to this outbreak.
The need to coordinate activities among a wide array of local, national and international laboratories and health agencies and law enforcement presented logistical challenges. A simultaneous criminal investigation was being conducted by the Garda National Drug Unit. Collaboration between law enforcement and public health in such an investigation was a novel activity. Law enforcement regulations required additional logistical steps in the handling and transportation of clinical specimens and heroin or other drug-related samples. All specimens and samples were considered evidence and had to follow a chain of custody procedure. Special licenses and permits were required for transportation to international reference laboratories.
At the time of this outbreak, clusters of unexplained illness were not reportable to Irish public health authorities. Without full investigative authority, case finding, medical record review, patient interviews and clinical and environmental specimen collection were often difficult. Regulations have now been amended for the reporting of infectious diseases in Ireland, and effective from 1 January 2004, unusual clusters or changing patterns of illness of public health concern must be reported to health authorities. This legislative change will help prevent delays in identification and investigation of future disease outbreaks. Furthermore, electronic disease reporting from emergency departments was not in place at the time of this outbreak. Implementation of an electronic surveillance system would potentially decrease the time to recognition of a cluster of unexplained illness and consequently should be addressed as a priority.
Between 2000 and 2004, there has been a dramatic increase in illness caused by spore-forming bacteria (Clostridium and Bacillus spp.) in IDUs in the United Kingdom, and reasons for this trend are unclear [28]. Understanding the unique medical, legal, and public health challenges faced when investigating these types of outbreaks will allow public health authorities and the medical community an opportunity to plan for future occurrences.
ACKNOWLEDGEMENTS
This investigation was a collaborative effort by the Greater Glasgow Health Board (Scotland), Scottish Centre for Infection and Environmental Health, Eastern Regional Health Authority (Ireland), National Disease Surveillance Centre (Ireland), Public Health Laboratory Service (United Kingdom), Centers for Disease Control and Prevention (USA) and numerous public health agencies and clinical institutions.
The authors thank: Liam English, Eric Mulvihill, Stephen Finn, Patricia O’Brien, Terry Boyle and Robert Quill, St. James’s Hospital, Dublin, Ireland; Eamon Leen, James Connolly Memorial Hospital, Dublin, Ireland; Tom O’Connell and Mary Cronin, Eastern Regional Health Authority, Dublin, Ireland; Marie Cassidy, Dublin City Coroner; Jane Salmon, Robert George, John Brazier, Public Health Laboratory System, United Kingdom; Scottish Centre for Infection and Environmental Control; Greater Glasgow Health Board, Scotland, UK; Jai Lingappa, Lisa Rotz, Ali Khan, Jordan Tappero, Bradley Perkins, David Ashford, Richard Meyer, Tanya Popovic, Sherif Zaki, Steve Jones, Anne Whitney, Harvey Holmes, David Whaley, Susan Maslanka and Jeannette Guarner, Centers for Disease Control and Prevention, Atlanta, GA, USA.
DECLARATION OF INTEREST
None.
REFERENCES
- 1.Greater Glasgow Health Board, SCIEH. Unexplained illness among drug injectors in Glasgow. Eurosurveill Wkly. 2000;4:20. [Google Scholar]
- 2.Eastern Regional Health Authority, National Disease Surveillance Centre – Dublin. Deaths from unexplained illness in heroin users in Dublin. Eurosurveill Wkly. 2000;4:22. [Google Scholar]
- 3.Djuretic T, Gill N. Serious unexplained illness among injecting drug users in England. Eurosurveill Wkly. 2000;4:23. [Google Scholar]
- 4.CDC. Unexplained illness and death among injecting-drug users – Scotland, Ireland and England, April–June 2000. Morb Mortal Wkly Rep. 2000;49:489–492. [PubMed] [Google Scholar]
- 5.Ringert SH, H⊘iby EA, Jensenius M et al. Injectional anthrax in a heroin skin-popper. Lancet. 2000;356:1574–1575. doi: 10.1016/s0140-6736(00)03133-0. [DOI] [PubMed] [Google Scholar]
- 6.Hoiby EA, Caugant DA. Systemic anthrax in an injecting drug user: Oslo, Norway, April 2000. Eurosurveill Wkly. 2000;4:19. [Google Scholar]
- 7.Brazier JS, Duerden BI, Hall V et al. Isolation and identification of Clostridium spp. from infections associated with the injection of drugs: experiences of a microbiological investigation team. J Med Microbiol. 2002;51:985–989. doi: 10.1099/0022-1317-51-11-985. [DOI] [PubMed] [Google Scholar]
- 8.Welch AR, Borriello SP, Barclay FE. Simplified procedure for tissue culture in routine detection of cytotoxins. J Clin Pathol. 1985;38:835–837. doi: 10.1136/jcp.38.7.835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.McLauchlin J, Salmon JE, Ahmed S et al. Amplified fragment length polymorphism (AFLP) analysis of Clostridium novyi, C. perfringens and Bacillus cereus isolated from injecting drug users during 2000. J Med Microbiol. 2002;51:990–1000. doi: 10.1099/0022-1317-51-11-990. [DOI] [PubMed] [Google Scholar]
- 10.McLauchlin J, Mithani V, Bolton FJ et al. An investigation into the microflora of heroin. J Med Microbiol. 2002;51:1001–1008. doi: 10.1099/0022-1317-51-11-1001. [DOI] [PubMed] [Google Scholar]
- 11.Jones JA, Salmon J, Djuretic T, Nichols G, George RC, Gill ON. An outbreak of serious illness and death among injecting drug users in England during 2000. J Med Microbiol. 2002;51:978–984. doi: 10.1099/0022-1317-51-11-978. [DOI] [PubMed] [Google Scholar]
- 12.McGuigan C, Penrice G, Gruer L et al. Lethal outbreak of infection with Clostridium novyi type A and other spore-forming organisms in Scottish injecting drug users. J Med Microbiol. 2002;51:971–977. doi: 10.1099/0022-1317-51-11-971. [DOI] [PubMed] [Google Scholar]
- 13.Finn SP, Leen E, English L, O’Briain DS. Autopsy findings in an outbreak of severe systemic illness in heroin users following injection site inflammation: an effect of Clostridium novyi exotoxin? Arch Pathol Lab Med. 2003;127:1465–1470. doi: 10.5858/2003-127-1465-AFIAOO. [DOI] [PubMed] [Google Scholar]
- 14.Simpson DD, Joe JW, Brown BS. Treatment retention and follow-up outcomes in the Drug Abuse Treatment Retention Study (DATOS) Psychol Addict Behav. 1997;11:294–307. [Google Scholar]
- 15.Cherubin CE, Sapira JD. The medical complications of drug addiction and the medical assessment of the intravenous drug user: 25 years later. Ann Intern Med. 1993;119:1017–1028. doi: 10.7326/0003-4819-119-10-199311150-00009. [DOI] [PubMed] [Google Scholar]
- 16.Binswanger IA, Kral AH, Bluthenthal RN, Rybold DJ, Edlin BR. High prevalence and abscesses among community-recruited injection drug users in San Francisco. Clin Infect Dis. 2000;30:579–581. doi: 10.1086/313703. [DOI] [PubMed] [Google Scholar]
- 17.Webb LOA, Schifano F, Cheeta S, Pollard M, Ghodse AH. Cause and manner of death in drug-related fatality: an analysis of drug-related deaths recorded by coroners in England and Wales in 2000. Drug Alcohol Depend. 2003;72:67–74. doi: 10.1016/s0376-8716(03)00191-1. [DOI] [PubMed] [Google Scholar]
- 18.Ward MBJ. Opiate-related deaths in Dublin. Ir J Med Sci. 2001;170:35–37. doi: 10.1007/BF03167718. [DOI] [PubMed] [Google Scholar]
- 19.Comiskey C, Barry J. A capture-recapture study of the prevalence and implications of opiate-use in Dublin. Eur J Public Health. 2001;11:198–200. doi: 10.1093/eurpub/11.2.198. [DOI] [PubMed] [Google Scholar]
- 20.CDC. Update: Clostridium novyi and unexplained illness among injecting-drug users – Scotland, Ireland and England, April–June 2000. Morb Mortal Wkly Rep. 2000;49:543–545. [PubMed] [Google Scholar]
- 21.Vlahov DSM, Astemborski J, Nelson KE. Bacterial infections and skin cleaning prior to injection among intravenous drug users. Public Health Rep. 1992;107:595–598. [PMC free article] [PubMed] [Google Scholar]
- 22.Fischer M, Whitney A, Ahmed S, Barry J, Jones J, Scheld M, Murray BE, Hughes JM. Emerging infections. Washington, DC: American Society for Microbiology Press; 2004. Unexplained severe illness and death among injecting drug users in Scotland, Ireland and England, April–August, 2000; pp. 122–132. [Google Scholar]
- 23.Mullen L, Barry J, Igoe D, Keenan E, Ward M, Murray K. Unexplained illnesses among injecting drug users in Dublin: a case control study. J Epidemiol Commun Health. 2002;56:575–576. doi: 10.1136/jech.56.8.575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Taylor A, Hutchinson S, Lingappa J et al. Severe illness and death among injecting drug users in Scotland: a case-control study. Epidemiol Infect. 2005;133:193–204. doi: 10.1017/s0950268804003504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.CDC. Tetanus among injecting-drug users California, 1997. Morb Mortal Wkly Rep. 1998;47:149–151. [PubMed] [Google Scholar]
- 26.Passaro DJ, Werner SB, McGee J, MacKenzie WR, Vugia DJ. Wound botulism associated with black tar heroin among injecting drug users. J Am Med Assoc. 1998;279:859–863. doi: 10.1001/jama.279.11.859. [DOI] [PubMed] [Google Scholar]
- 27.Werner SB, Passaro D, McGee J, Schechter R, Vugia DJ. Wound botulism in California, 1951–1998: recent epidemic in heroin injectors. Clin Infect Dis. 2000;31:1018–1024. doi: 10.1086/318134. [DOI] [PubMed] [Google Scholar]
- 28.Brett MM, Hood J, Brazier JS, Duerden BI, Hahne SJM. Soft tissue infections caused by spore-forming bacteria in injecting drug users in the United Kingdom. Epidemiol Infect. 2005;133:575–582. doi: 10.1017/s0950268805003845. [DOI] [PMC free article] [PubMed] [Google Scholar]