SUMMARY
In September 2000, haemolytic uraemic syndrome (HUS) was diagnosed in a 10-month-old child with a prodromal history of vomiting and diarrhoea (non-bloody). Investigation revealed that a self-limiting gastrointestinal illness (mean duration 48 h) had occurred among immediate and extended family in the 2 weeks prior to the child’s admission. The epidemiology of the illness suggested person-to-person spread. Five children (close family contacts) had E. coli O26 verocytotoxin (VT1 and VT2) isolated from stools. Stool culture and serology from the index case were negative for shiga toxin-producing E. coli (STEC) organisms. Control measures in accordance with the Public Health Laboratory Service (PHLS), verocytotoxogenic organisms (VTEC) guidelines were applied to prevent further spread among the extended family and contacts. Despite detailed food and environmental exposure histories, the source of the illness was not identified. This incident highlights the importance of investigation of cases of post-diarrhoeal HUS, for potential shiga toxin E. coli aetiology.
INTRODUCTION
Karmali and colleagues first identified the association between haemolytic uraemic syndrome (HUS) and infection with Shiga toxin-producing E. coli (STEC) O157:H7 in children [1, 2]. Recently, other STEC organisms especially O26 have emerged as significant causes of HUS [3–5]. Non-O157 STEC is often missed in routine laboratory analysis of stool specimens due to a lack of standardized methodology [6, 7]. A study assessing the distribution of STEC serotypes causing HUS in Germany and Austria found that non-O157: H7 serotypes were the most prevalent serotype in children up to 12 months of age [8]. The first gastroenteric outbreak caused by non-O157 STEC in Ireland was described by McMaster et al. [9]. We describe post-diarrhoeal HUS associated with a cluster of cases caused by STEC O26 in Ireland.
MATERIALS AND METHODS
Initial investigation and control measures
A case of HUS was notified on 6 September 2000 in a 10-month-old female to Public Health. Initial investigation revealed that one of her siblings had sought medical attention for diarrhoea 16 days previously. Another sibling, two cousins and an aunt also had a recent history of diarrhoea. Children under 5 years were excluded from returning to school after the summer holidays until negative stool sample results were obtained [10]. Advice was given with respect to enteric precautions and on the necessity for medical attention if symptomatic [10]. A multidiscplinary outbreak control team was convened to further investigate the suspicion of a cluster of cases and to coordinate control measures.
Epidemiological investigation
The index case had developed HUS following a 72-h history of vomiting and diarrhoea (non-bloody). She was hospitalized for 16 days, necessitated haemodialysis, and made a full recovery. Detailed collateral history-taking revealed close interaction between three households comprising seven adults and seven children in total. The first household (no. 1) comprised the index case, her two brothers aged 2·5 and 5 years and their parents. The second household (no. 2) comprised her four cousins aged, 2, 4, 6 and 8 years and their parents (the mothers in household nos. 1 and 2 were sisters). The third household (no. 3) comprised the maternal grandparents and their daughter (sister of mothers of household nos. 1 and 2). Detailed demographic and illness histories, along with stool samples were obtained from the three households.
Microbiological investigation
Initial stool samples from household no. 1 were negative for enteric pathogens including E. coli O157 in the paediatric hospital. Their stools were forwarded for STEC studies (O157 and non-O157) to the Regional Public Health Laboratory (RPHL) given the high-risk clinical history of the index case. The stools from household nos. 2 and 3 went directly to the RPHL. Stool samples were inoculated in parallel onto (1) Cefixime and Tellurite Sorbitol MaConkey (CT–SMAC), (2) Tryptone soy broth (TSB) and (3) MaConkey agar. All were incubated overnight aerobically at 37°C. The TSB broth was subcultured onto CT–SMAC. All CT–SMAC plates were examined for non-sorbitol (colourless) colonies. Any suspicious colonies had confirmatory biochemical identification using API 20E strips, and nutrient agar plates were utilized for agglutination tests with E. coli O157 antisera and non-O157 antisera. The E. coli antisera currently utilized in RPHL, are outlined in the Table. Any positive agglutinates had confirmatory biochemical identification as above. All probable STEC organisms at that time were sent to the Public Health Laboratory Services, Colindale, UK for confirmation of verocytotoxin status and phage typing.
Table.
E. coli antisera held in the Regional Public Health Laboratory, Health Service Executive South Western Area, Dublin

Individual antisera is held for all the above serogroups and in addition for serotypes O18, O103, and O145.
Environmental investigation
Food histories and relevant environmental exposure histories were carried out on the three households.
RESULTS
Epidemiological results
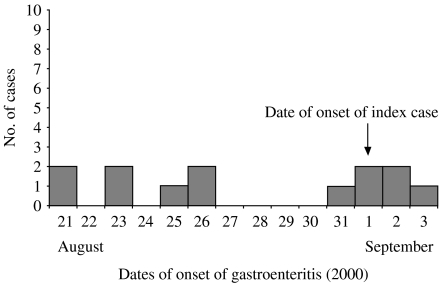
Six of the seven adults suffered either vomiting or diarrhoea, two preceding the index case’s illness onset date. All seven children had a gastrointestinal illness (mean duration 48 h) of whom two received medical attention (the index case and her brother). All illness among the children preceded the date of onset of the index case but one cousin had a relapse on 10 September 2000. The dates of onset of illness are outlined in the Figure. From alerts issued to General Practitioners (GPs), there was no evidence of an increase in gastrointestinal illness in the community.
Fig.
Epidemic curve of the cluster of E. coli O26 gastroenteritis.
Microbiological results
Five children (two siblings and three cousins of the index case) had E. coli O26 verocytotoxin (VT1 and VT2) isolated. Haematological indices of those culture-positive were normal. One cousin aged 4 years and all seven adults were negative for STEC organisms. Another of the cousins aged 2 years was initially negative, however, repeat samples, due to her persistent gastroenteric symptoms were subsequently positive for E. coli O26. She achieved microbiological clearance 3 weeks later. Stool samples from the index case were culture negative for STEC organisms. She also had negative serology for E. coli O157 and O26.
Environmental results
No identifiable risk exposures were noted.
Further control measures
On notification of positive stool cultures for E. coli O26 from the two cousins (household no. 2), the parents of the index case (household no. 1) were requested to continue to keep her two siblings out of school pending microbiological analysis of their stools for E. coli O26 (which were subsequently positive), as they were in ‘Risk groups 3 and 4’ [10]. Local risk assessment warranted further interventions:
Household 1: Exclusion of both siblings (Risk groups 3 and 4) of the index case from special care facilities until microbiological clearance was obtained.
Household 2: Voluntary exclusion of the two culture-positive children aged 6 and 8 years from school pending an assessment of the school facilities, their ability to practise good hygiene and subsequent microbiological clearance. Advice was also given to ensure that the positive toddler (age 2 years) did not attend childcare facilities or gatherings until microbiological clearance was achieved.
Patient STEC management advice was discussed with the relevant GPs.
Enteric precautions and advice on environmental decontamination were issued to all three households.
Relevant national public health authorities were regularly updated.
An alert was issued to local GPs and all consultant microbiologists in the region, of the occurrence of the cluster and of the need for prompt notification of other such cases.
DISCUSSION
This is the first case of HUS associated with E. coli O26 notified in the Republic of Ireland. E. coli O26 was one of the first strains of E. coli to be considered a cause of infantile diarrhoea [11]. Recent evidence suggests that cattle and their products are the predominant source of this serotype [12, 13]. In 2001, there were four cases of E. coli O26 notified in Ireland, followed by one in 2002 and four in 2003 (one of which had HUS). To our knowledge, no further clusters of E. coli O26 have occurred since this cluster.
The index case had non-bloody diarrhoea. This may be consistent with the opinion that non-O157 STEC are less likely to be associated with bloody stools [14]. The organism was not isolated from the index case despite a number of attempts, which is consistent with HUS being a post-infection complication. A Czechoslovakian study found that STEC organisms were cultured in only 46% (16/35) of children with HUS and advised that detection of specific anti-LPS antibodies in acute phase sera is the most sensitive diagnostic method of STEC infection in HUS [15]. Screening for such antibodies was negative in this child.
There is minimal information on the public health significance of duration of excretion by cases of E. coli O26. One cousin took 3 weeks for microbiological clearance. Previous lengths of shedding reported for STEC O26 range from <6 days to 13 days [16] and 2–5 weeks [9]. No source was found for this cluster of infection, but the epidemiology suggested person-to-person spread. This mode of spread from mildly symptomatic cases or asymptomatic carriers may have a major role in the epidemiology of secondary cases of HUS [17].
Tarr & Neill [18], along with the Scottish Task Force [19] suggest categories of patients whom should be tested for non-O157. If E. coli O26 had not been diagnosed in this cluster, the siblings of the index case who fitted two risk groups [10] would have returned after the summer holidays to their special needs schools, while still excreting a STEC organism. The potential to spread this virulent pathogen is high among such vulnerable groups.
The laboratory diagnosis of non-O157 E. coli infections from clinical samples is not standardized. In Ireland the use of immunomagnetic separation and molecular techniques (PCR) for verocytotoxin genes is minimal. Since 2003 the RPHL, South Western Area Health Board offers a national molecular (PCR) diagnostic service for O157 and non-O157 E. coli infections. However, there has probably been significant under-diagnosis of such infections to date.
Even though STEC infection was not notifiable in Ireland at the time of this cluster, this incident highlights the importance of notifying post-diarrhoeal HUS to Public Health Authorities. STEC infection has subsequently become notifiable in Ireland with an amendment to the Infectious Disease Regulations in December 2003 [20]. Currently there are no Irish standardized public health guidelines on the notification and investigation of a case of post-diarrhoeal HUS. We would recommend that such guidelines be drawn up. The Health Protection Surveillance Centre (HPSC) has convened a sub-committee to consider the management, investigation, and epidemiology of STEC infection. We welcome this initiative, which will standardize the management of STEC infections in Ireland.
ACKNOWLEDGEMENTS
The authors acknowledge the following: the area medical officers and environmental health officers who took part in the investigation of this cluster; the laboratory staff in the paediatric hospital and in the Regional Public Health Laboratory; the relevant staff at PHLS, Colindale; the families concerned and their GPs; the educational professionals and the healthcare professionals in the facilities that the children attended; support staff in the Area Health Board and the Department of Public Health; Ms Eilish Creamer, infection control nurse (formerly of the Department of Public Health); Dr Lelia Thornton for her helpful advice in management of the case; Dr Marie Laffoy and Dr Brian O’Herlihy – Deputy and Director of Public Health for their support during the investigation of this cluster, and Ms. Patricia Garvey of HPSC, Dublin.
DECLARATION OF INTEREST
None.
REFERENCES
- 1.Karmali MA, Steele BT, Petric M, Lim C. Sporadic cases of haemolytic uraemic syndrome associated with faecal cytotoxin and cytotoxin producing Escherichia in stools. Lancet. 1983;1:619–620. doi: 10.1016/s0140-6736(83)91795-6. [DOI] [PubMed] [Google Scholar]
- 2.Karmali MA, Petric M, Lim C, Fleming PC, Arbus GS, Lior H. The association between idiopathic haemolytic uraemic syndrome and infection by verotoxin-producing Escherichia coli. J Infect Dis. 1985;151:775–782. doi: 10.1093/infdis/151.5.775. [DOI] [PubMed] [Google Scholar]
- 3.Johnson RP, Clarke RC, Wilson JB et al. Growing concerns and recent outbreaks involving non-O157:H7 serotypes of verotoxigenic Escherichia coli. J Food Prot. 1996;59:1112–1122. doi: 10.4315/0362-028X-59.10.1112. [DOI] [PubMed] [Google Scholar]
- 4.Zhang W-L, Bielaszewska M, Liesegang A et al. Molecular characteristics and epidemiological significance of Shiga toxin-producing Escherichia coli O26 strains. J Clin Microbiol. 2000;38:2134–2140. doi: 10.1128/jcm.38.6.2134-2140.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Misselwitz J, Karch H, Bielaszewska M, John U, Ringelmann F, Ronnefarth G, Patzer L. Cluster of haemolytic uraemic syndrome caused by Shiga toxin producing Escherichia coli O26:H11. Paediatr Infect Dis J. 2003;22:349–354. doi: 10.1097/01.inf.0000059338.38673.ae. [DOI] [PubMed] [Google Scholar]
- 6.World Health Organisation. Geneva, Switzerland: Geneva: WHO; 1997. Food Safety Unit Consultations and Workshops. Prevention and control of enterohaemorrhagic Escherichia coli (EHEC) infections. . Report of a WHO consultation; , 28 April–1 May. ; 1997. Report No: WHO/FSF/FOS/97.6. [Google Scholar]
- 7.Tarr PI. Escherichia coli O157:H7. Clinical, diagnostic and epidemiological aspects of human infection. Clin Infect Dis. 1995;20:1–10. doi: 10.1093/clinids/20.1.1. [DOI] [PubMed] [Google Scholar]
- 8.Verweyen HM, Karch H, Allerberger F, Zimmerhackl LB. Enterohaemhorragic Escherichia coli (EHEC) in Paediatric Haemolytic Uraemic Syndrome: a prospective study in Germany and Austria. Infection. 1999;6:341–347. doi: 10.1007/s150100050040. [DOI] [PubMed] [Google Scholar]
- 9.McMaster C, Roch EA, Willshaw GA, Doherty A, Kinnear W, Cheasty T. Verocytotoxin producing Escherichia coli serotype O26:H11 outbreak in an Irish crèche. Eur J Clin Microbiol Infect Dis. 2001;20:430–432. doi: 10.1007/pl00011285. [DOI] [PubMed] [Google Scholar]
- 10.PHLS Advisory Committee on Gastrointestinal Infections. Guidelines for the control of infection with Verocytotoxin producing Escherichia Coli (VTEC) Commun Dis Public Health. 2000;3:14–23. [PubMed] [Google Scholar]
- 11.Scotland SM, Willishaw GA, Smith HR, Rowe B. Properties of Escherichia coli O26:H11 in relation to their enteropathogenic or enterohaemorrhagic classification. J Infect Dis. 1990;166:1069–1074. doi: 10.1093/infdis/162.5.1069. [DOI] [PubMed] [Google Scholar]
- 12.Bettelheim KA. Role of the non-O157 VTEC. J Appl Microbiol Symposium. 2000;88:38S–50S. doi: 10.1111/j.1365-2672.2000.tb05331.x. [DOI] [PubMed] [Google Scholar]
- 13.Bettelheim KA. Non-O157 Verotoxin producing Escherichia coli: a problem, paradox and paradigm. Exp Biol Med. 2003;4:333–344. doi: 10.1177/153537020322800402. [DOI] [PubMed] [Google Scholar]
- 14.Griffin PM, Tauxe RV. The epidemiology of infection caused by Escherichia coli O157:H7, other enterohaemorrhagic E. coli and the associated haemolytic uremic syndrome. Epidemiol Rev. 1991;13:60–98. doi: 10.1093/oxfordjournals.epirev.a036079. [DOI] [PubMed] [Google Scholar]
- 15.Bielaszewska M, Janda J, Blahova K, Feber J, Potuznik V, Souckova A. Verocytotoxin producing Escherichia coli in children with haemolytic uraemic syndrome in the Czech Republic. Clin Nephrol. 1996;1:42–44. [PubMed] [Google Scholar]
- 16.Hiruta N, Murase T, Okamura N. An outbreak due to multiple antimicrobial resistant Shiga toxin-producing Escherichia coli O26:H11 in a nursery. Epidemiol Infect. 2000;127:221–227. doi: 10.1017/s0950268801006069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tozzi AE, Caprioli A, Minella F et al. Shiga toxin-producing Escherichia coli infections associated with Haemolytic Uraemic Syndrome, Italy, 1998–2000. Emerg Inf Dis. 2003;9:106–108. doi: 10.3201/eid0901.020266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tarr PI, Neill MA. The problem of non-O157:H7 Shiga Toxin (Verocytotoxin) producing Escherichia coli. J Infect Dis. 1996;174:1136–1139. doi: 10.1093/infdis/174.5.1136. [DOI] [PubMed] [Google Scholar]
- 19.Scottish Task Force on E. coli O157. June 2001.
- 20.Infectious Diseases (Amendment) (No. 3) Regulations 2003. Stationery Office; Dublin: Statutory Instrument No. 707). Irish Statute Book, Government of Ireland. [Google Scholar]