SUMMARY
Fresh produce is an important part of a healthy diet and is consumed in greater quantity in the United States than ever before. Consumption of cantaloupe has recently been associated with several large outbreaks of infections in North America, highlighting the need for a better understanding of practices and processes that may contribute to contamination. We reviewed all cantaloupe-associated outbreaks that were reported to the Centers for Disease Control and Prevention (CDC) and published in the literature. Twenty-three outbreaks occurred between 1984 and 2002; 1434 people became ill, 42 were hospitalized, and two died in these outbreaks. Aetiological agents in the outbreaks included five serotypes of Salmonella enterica, Campylobacter jejuni, Escherichia coli O157:H7, and norovirus. We reviewed processes contributing to cantaloupe contamination, conditions affecting survival and growth of bacterial pathogens on melons, and potential methods for sanitization. For maximum safety, industry, federal, and international partners must collaborate to ensure that appropriate interventions are in place to minimize the risk of contamination and prevent the growth of pathogens during cantaloupe production, processing, storage, and preparation.
INTRODUCTION
Fresh produce has been implicated in outbreaks of foodborne illness in the United States with increased frequency in the past two decades [1–5]. Several factors may be contributing to this trend. Produce is now available all year round as a result of global marketing and trade, and international travel and restaurant dining may enhance the likelihood of consumer exposure to contaminated produce [3, 6–8]. In addition, fresh fruits and vegetables have been promoted to consumers as an important part of a healthy lifestyle. Numerous campaigns have highlighted the nutritional value of produce, resulting in an increase in per capita consumption.
Cantaloupe (Cucumis melo var. reticulatus) is among the fresh fruits that are being consumed in larger quantities in recent years. The annual US per capita consumption of cantaloupe increased from 5·8 lb in 1980 to 11·3 lb in 2002 [9]. Recently, several large outbreaks of Salmonella enterica serotype Poona infections were associated with consuming cantaloupes, highlighting the need for enhancing cantaloupe safety and resulting in importation restrictions for implicated producers [10, 11]. We reviewed the Centers for Disease Control and Prevention (CDC) reports of outbreaks of foodborne infections from 50 states and four territories, as well as the global literature, to summarize information on cantaloupe-associated outbreaks, including aetiological agents, possible sources of contamination, conditions affecting survival and growth of pathogens on melons, and procedures for pathogen reduction or elimination.
METHODS
We searched PubMed for all outbreaks that reported cantaloupe or muskmelon as the vehicle of infection because muskmelon is the common name for cantaloupe in some regions of the United States. Outbreaks that implicated cantaloupe or muskmelon in addition to another food item were included in the analysis; however, outbreaks that implicated honeydew or casaba melons alone were not included.
We defined an outbreak as two or more epidemiologically linked cases of illness. Not all outbreak-associated cases had laboratory-confirmed infection. Investigators identified food vehicles that were statistically significantly associated with illness or that yielded the aetiological agent when cultured. Aetiological agents were identified by laboratory confirmation from two or more case-patients in each outbreak.
Data sources included the CDC foodborne outbreak surveillance system, which collected reports of foodborne outbreaks from 50 US states and four territories between 1973 and 2003, and outbreak reports from the literature. The CDC foodborne outbreak surveillance data collection form was modified in 1998 to capture demographic information, such as gender and age of case patients, thus providing additional data for the 1998–2003 period. We searched PubMed with the key words ‘cantaloupe’ or ‘melon’ in combination with ‘epidemiology’, ‘outbreak’, or ‘illness’. Bibliographies of resulting articles were also searched.
We also reviewed the scientific literature for cantaloupe and produce microbiological studies. PubMed search terms included ‘cantaloupe’ or ‘produce’ in combination with ‘microbiology’. Additionally, bibliographies and personal files were reviewed. We assumed findings from studies involving non-melon produce could also have relevance to melons.
RESULTS
We identified 25 outbreaks associated with consumption of cantaloupes reported to the CDC foodborne outbreak surveillance system between 1973 and 2003 (Table 1). Three additional outbreaks were discovered in a search of the literature (outbreaks 2, 9, 13 in Table 1). Each outbreak identified raw cantaloupe as the vehicle of infection. Muskmelon was not implicated in any outbreak. In 14 outbreaks, food items in addition to cantaloupe were implicated as vehicles. In all, 1615 people were reported ill (some reports included only the lower limit of the number ill), 57 were hospitalized, and two died.
Table 1.
Cantaloupe-associated outbreaks in the United States and Canada, 1973–2003
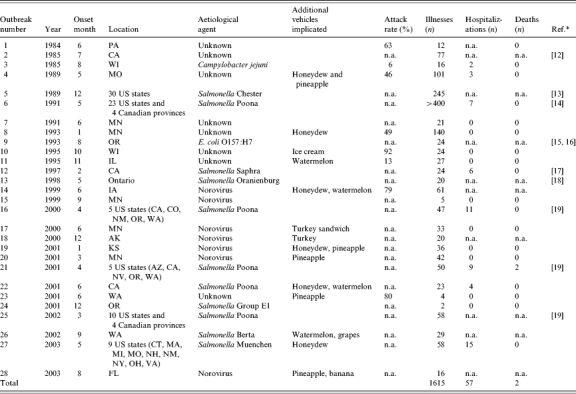
Other than outbreaks 2, 9, and 13, all outbreaks were identified in the CDC foodborne outbreak surveillance database.
Seven serotypes of S. enterica (Berta, Chester, Muenchen, Oranienburg, Poona, Saphra, and an unknown serotype from Group E1) accounted for 11 (39%) of the outbreaks, an estimated 956 (59%) of the cases, 52 (91%) of the hospitalizations, and two deaths. Norovirus caused an additional seven (25%) outbreaks, and Campylobacter jejuni and Escherichia coli O157:H7 each caused one (4%) outbreak. No aetiological agent was determined in eight (29%) of the outbreaks, although the carbamate pesticide, Aldicarb, was suspected in one [12].
Analysis of 1998–2003 data revealed that more females than males developed illness. Most cases occurred in adults, with only 10% of case-patients being <19 years old and none aged <1 year. Case-patients resided in the United States and Canada. No outbreaks from other geographic areas were identified (Table 1).
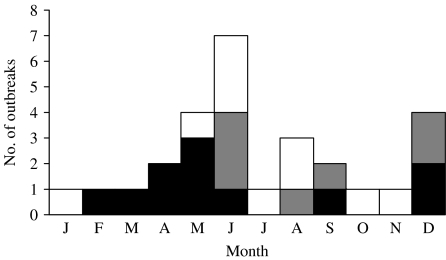
Of the 28 outbreaks, 19 (68%) were reported between 1994 and 2003, the final decade of the 30-year surveillance period. To evaluate the effect of changes in surveillance, we examined the proportion of outbreaks associated with cantaloupe before and after 1998. Of foodborne outbreaks with a known vehicle, cantaloupe was implicated in 12 (0·25%) of the 4770 that occurred between 1973 and 1997, and 16 (0·34%) of the 4721 that occurred between 1998 and 2003. At least one outbreak occurred in each calendar month, while outbreaks of salmonella infections occurred in December (2), February (1), March (1), April (2), May (3), June (1), and September (1) (Fig. 1).
Fig. 1.
Number of cantaloupe-associated outbreaks, by aetiology and month. □, Other;
 , norovirus; ■, salmonella.
, norovirus; ■, salmonella.
Seventeen (61%) of the 28 outbreaks were associated with cantaloupe prepared in a restaurant or by a caterer. Four (14%) additional outbreaks were associated with cantaloupe prepared in a grocery store. Results of environmental investigations were reported irregularly and inconsistently. Findings included contaminated preparation equipment, poor food-handler hygiene, ill food handlers, and temperature abuse. Four of the seven outbreaks caused by norovirus were reported to involve ill food handlers. Information about the locations at which cantaloupes were grown was not recorded for most outbreaks.
DISCUSSION
Sources of contamination
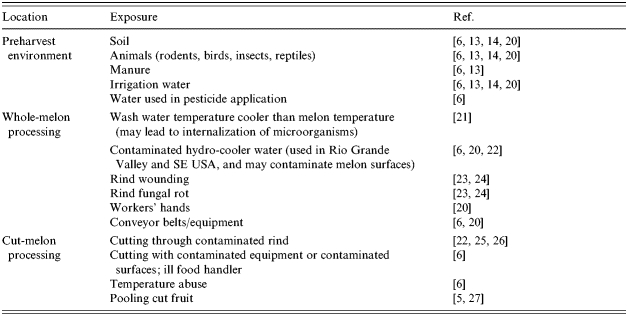
Cantaloupes may become contaminated before harvest, during harvesting, packing, and storage, and during processing or preparation of cut products (Table 2).
Table 2.
Risk factors for produce contamination
Preharvest environment
Soil and soil amendments such as improperly composted manure, contaminated irrigation water, wild and domestic animals, and farm workers are potential vehicles of contamination of preharvest melons [8, 28, 29]. Microorganisms capable of causing human diseases can survive in soil for protracted durations. Listeria monocytogenes can survive in soil for at least 8 weeks [30], salmonella and E. coli O157:H7 can survive up to 23 weeks, and viruses can live for 3 weeks [31–33]. These pathogens may also be introduced by infected or colonized wild animals, such as reptiles, birds, and rodents, eating fruit and defecating directly in fields [29], and further distributed by insects [34] and perhaps nematodes [35]. Poor field-worker hygiene could also contribute to surface contamination [29, 36]. In addition, the netting that naturally covers the surface of cantaloupe rinds (Fig. 2) may facilitate attachment and survival of microorganisms from the soil or irrigation water [37]. Evidence suggests that some preharvest fruits and vegetables may become internally contaminated with enteric pathogens through unknown mechanisms [28, 38, 39]; this may also occur in melons.
Fig. 2.
Scanning electron micrograph of Cucumis melo var. reticulatus rind surface showing porous tissue structure (Courtesy of Janice Carr, CDC.).
Whole melon handling and storage
Although the aim of post-harvest washing is largely to remove soil and clean the melons, it may also contribute to contamination. In packing houses, fresh produce can harbour microorganisms at populations of 104–106 c.f.u./g [19, 40, 41]. For apples and tomatoes, a negative temperature differential (i.e. the temperature of the wash water is lower than the temperature of the fruit) has been shown to enhance infiltration of microorganisms into subsurface tissue of the fruit [42, 43]. Similarly, if cantaloupes are submerged in cooler water or hydrocooled, hydrostatic pressure differences may facilitate infiltration of salmonella into the rind surface of some varieties [21]. Enteric microorganisms have been found in wash and hydrocooler water at several cantaloupe harvesting locations and packing facilities [37]. This problem may be further complicated by regional practices and environmental conditions. For example, many growers in the Western United States have adopted best management practices for cantaloupe that avoid exposing the product to water [37]. However, in some regions, there may be a greater need for washing and/or fungicide application to clean cantaloupes and maintain the quality of the product.
Pathogens may survive and even grow on the surface of intact melons during shipment and storage (Table 3). S. Poona survives on intact cantaloupe rind for up to 14 days [23, 45] and grows in cantaloupe wounds [24, 46]. E. coli O157:H7 populations on intact cantaloupe rind can increase by two logs within 4 days at 25°C [15]. Cantaloupes are also susceptible to post-harvest fungal rots, especially when stored unrefrigerated and at a high relative humidity [22]. Migration of S. Poona into the interior of the cantaloupe, followed by growth, is enhanced by co-infection with some species of moulds [24]. These observations point out the need to discard the entire melon when only a small area shows visual decay.
Table 3.
Observations of survival and growth of foodborne pathogens on melons
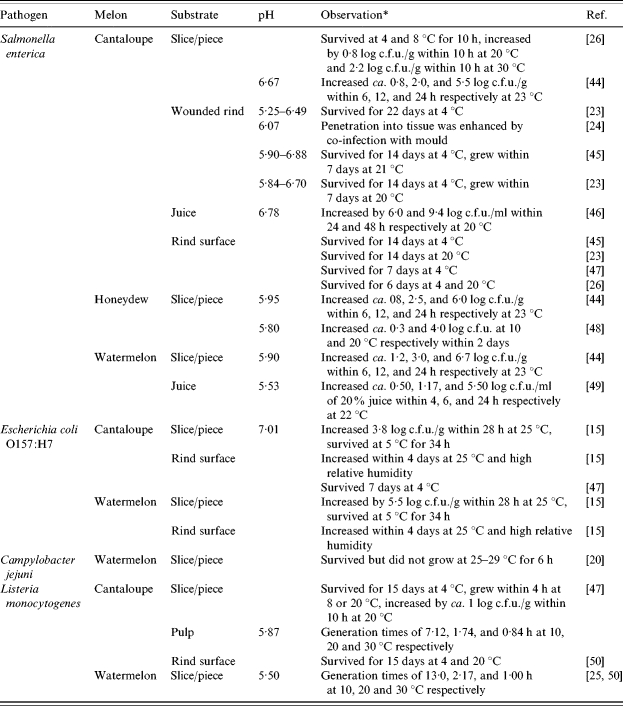
Hours or days noted for survival indicate that pathogens survived for at least these lengths of time.
Cut melon processing
Barring a negative temperature differential between wash water and cantaloupes, and in the absence of mould growth, the physically intact rind comprises a natural barrier to infection. Even if the rind is contaminated, the edible cantaloupe flesh should remain free of enteric pathogens until the rind is breached [15, 25, 26]. The introduction of salmonella from the exterior surface to the internal flesh of cantaloupes and other fruits has been documented [22, 26, 51, 52]. Epidemiological and laboratory evidence in an outbreak of E. coli O157:H7 infections associated with cut cantaloupe suggested that contamination may occur not only by cutting through contaminated rinds but also by cutting with contaminated utensils [16].
Storage of cut cantaloupes at temperatures that are not adequately cool exacerbates the safety risk because juice released by cut tissues is a good growth medium for foodborne pathogens (Table 3). Cut melons are often displayed in grocery stores, farmers’ markets, and salad bars on ice, but the surface of the pieces may be at a temperature close to ambient [53]. In much the same way that ground beef, representing a composite of meat from many carcasses, is more likely to internally harbour pathogens than would a single cut of steak, pooling cut melon pieces exacerbates the potential for contamination of larger quantities [5].
Mitigation of contamination
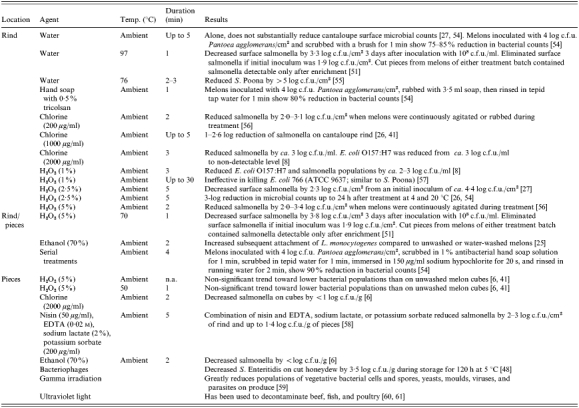
Several methods of reducing pathogens on intact and cut cantaloupes have been attempted with varying degrees of success (Table 4). Washing the rind surface of cantaloupes with water or sanitizers does not always substantially reduce microbial counts [8, 25, 37, 41, 62]. Treatment of melon flesh with food-safe sanitizers also does not eliminate bacteria. Sanitizers evaluated have included hydrogen peroxide, sodium hypochlorite (chlorine), and ethanol. These sanitizers are not effective in eliminating pathogens, in part because organic materials in melon tissues neutralize the bactericidal activity [6, 8]. Bacteriophages, gamma irradiation, and ultraviolet light treatment are possible alternatives to sanitizing melons but have not been sufficiently evaluated for their efficacy. Direct application of sanitizers to cut cantaloupe poses an additional concern. Reduction or elimination of microorganisms that would otherwise successfully compete with foodborne pathogens may lead to overgrowth of pathogens that might survive the treatment. Following treatment of endive with 10% hydrogen peroxide, for example, populations of L. monocytogenes actually increased [63].
Table 4.
Efficacy of treatments to decontaminate cantaloupes
CONCLUSIONS
Outbreaks of illness associated with cantaloupe consumption in the United States have not been rare in recent years. We identified no single microorganism or obvious mode of contamination that appeared to be the cause of this trend. Instead, cantaloupes are susceptible to contamination in multiple ways, including internalization of bacteria through intact or damaged rind tissue and contact with contaminated surfaces during processing or preparation. In addition, cantaloupes may become contaminated if they are grown, harvested, or packed in areas where hygienic practices are less than adequate. Effective methods for decontaminating whole and cut melons have not been identified. Efforts to reduce cantaloupe-associated illness are needed by growers, processors, and food preparers.
Although we report more than 1600 cases of illness associated with cantaloupe consumption in the United States and Canada during the past 30 years, the true burden of foodborne disease associated with cantaloupes is probably much greater. While most of the outbreaks we report (Table 1) were associated with cantaloupe prepared at commercial eating establishments, contamination can occur at any point along the supply chain. In addition, a large number of cantaloupe-related illnesses also probably occurred among clusters too small to be detected easily. Outbreaks also comprise a small proportion of all episodes of foodborne illness each year; thus, a large number of sporadic cases of illness associated with cantaloupe consumption probably occurred between 1973 and 2003 that are not included in our estimates [64]. Further, we restricted analysis to outbreaks which specifically named cantaloupe or muskmelon as a vehicle of infection; a number of additional outbreaks associated with fruit salad, which may contain cantaloupe, were excluded.
Since 1994, outbreaks of infections associated with cantaloupe consumption have been reported with increased frequency. Data from these outbreaks are consistent with other data suggesting an increase in outbreaks associated with consumption of fresh produce and outbreak reporting in general. Data also correspond to an increase in consumption of cantaloupes and raw produce, and increased availability of produce all year round from global distributors. It is possible that awareness about cantaloupes and other produce as potential vehicles of pathogens has increased among investigators in recent years. If this is so, the documented recent increase in the number of cantaloupe-associated outbreaks might be misleading; however, the total number of outbreaks associated with cantaloupes may have been greatly underestimated during the period 1973–2003.
In 1998, the US Food and Drug Administration (FDA) issued voluntary guidelines for good agricultural practices (GAPs) and good manufacturing practices (GMPs) for growing and packing fresh produce [65]. Several documents outlining pre- and post-harvest guidelines for microbiological safety of fresh and fresh-cut produce, including cantaloupes, have been published [40, 66–70]. These guidelines are directed, in part, toward reducing safety risks associated with cantaloupes.
We found that illness associated with consumption of cantaloupes occurred throughout the year. Because the US cantaloupe production season typically spans from May to October, outbreaks were probably associated with both domestic and imported cantaloupes. Both domestic and imported product samples have also yielded microbiological evidence of contamination in testing by regulatory agencies. In 1999, the FDA cultured eight different imported produce items and found that cantaloupe was the third most commonly contaminated item, with 7·3% of samples yielding salmonella or shigella [40]. In a similar survey of domestic produce in 2000, cantaloupe was the second most commonly contaminated item; 3·0% of cantaloupes yielded salmonella or shigella [71].
As a result of the 1999 FDA survey, seven Central American and Mexican firms were placed on detention without physical examination (DWPE), requiring importers to prove that their produce was not grown under conditions likely to lead to adulterated product [11, 19]. In response to continued findings of positive product samples, observations of irregularities during inspections, and additional outbreaks of salmonellosis associated with cantaloupes grown in Mexico, an Import Alert was issued to include all Mexican cantaloupe growers in October 2002 [10]. Firms that believe that their practices and conditions are adequate may submit information to FDA and request removal from the Import Alert; a number of firms have done this. Because complete outbreak data are not yet available for 2004–2005, it is unknown if these measures have affected the number of cantaloupe-associated illnesses. During future outbreak investigations, collecting information about cantaloupe source might greatly facilitate regulatory response.
What steps can consumers attempting to follow a healthy diet take to minimize the risk of illness associated with potentially contaminated cantaloupes? The FDA has recommended that consumers avoid produce with blemishes, wash hands with soap before handling melons, and scrub melons with a brush under cool tap water before consumption [40]. As with any food preparation, utensils, knives, and cutting boards should be cleaned in hot, soapy or chlorinated water before and after use, and should not be cross-contaminated with uncooked foods, particularly those of animal origin. Intact cantaloupes may be stored at room temperature, but cut products should be refrigerated within 2 h of preparation, or else discarded. Even with these precautions, some level of risk of illness associated with the consumption of cantaloupes remains. A successful strategy to improve the safety of melons will require education, regulation, cooperation, and commitment throughout the supply chain and at the industrial, national, and international levels.
ACKNOWLEDGEMENTS
We thank Clifford Purdy for sharing his technical expertise.
DECLARATION OF INTEREST
None.
REFERENCES
- 1.Bean N, Griffin P. Foodborne disease outbreaks in the United States, 1973–1987: pathogens, vehicles, and trends. J Food Prot. 1990;53:804–817. doi: 10.4315/0362-028X-53.9.804. [DOI] [PubMed] [Google Scholar]
- 2.Beuchat L. Ecological factors influencing survival and growth of human pathogens on raw fruits and vegetables. Microbes Infect. 2003;4:413–423. doi: 10.1016/s1286-4579(02)01555-1. [DOI] [PubMed] [Google Scholar]
- 3.Institute of Food Technologists. 2001. www.cfsan.fda.gov/~comm/ift3-toc.html. www.cfsan.fda.gov/~comm/ift3-toc.html Analysis and evaluation of preventative control measures for the control and reduction/elimination of microbial hazards on fresh and fresh-cut produce. Report of the IFT for the US Food and Drug Administration of the US Department of Health and Human Services. ). Accessed 27 October 2003.
- 4.Sivapalasingam S, Friedman C, Mackinnon L, Tauxe R. Fresh produce: a growing cause of outbreaks of foodborne illness in the United States, 1973–1997. J Food Prot. 2004;67:2342–2353. doi: 10.4315/0362-028x-67.10.2342. [DOI] [PubMed] [Google Scholar]
- 5.Tauxe R, Kruse H, Hedberg C, Potter M, Madden J, Wachsmuth K. Microbial hazards and emerging issues associated with produce: a preliminary report to the National Advisory Committee on microbiological criteria for foods. J Food Prot. 1997;60:1400–1408. doi: 10.4315/0362-028X-60.11.1400. [DOI] [PubMed] [Google Scholar]
- 6.Beuchat L, Ryu J. Produce handling and processing practices. Emerg Infect Dis. 1997;3:459–465. doi: 10.3201/eid0304.970407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.National Advisory Committee on Microbiological Criteria for Foods. Microbiological safety evaluations and recommendations on fresh produce. Food Control. 1999;10:117–143. [Google Scholar]
- 8.Park C, Beuchat L. Evaluation of sanitizers for killing Escherichia coli O157:H7, Salmonella, and naturally occurring microorganisms on cantaloupes, honeydew melons, and asparagus. Dairy, Food and Enviornmental Sanitation. 1999;19:842–847. [Google Scholar]
- 9.US Department of Agriculture http://www.ers.usda.gov/publications/vgs/jun03/vgs297.pdf. http://www.ers.usda.gov/publications/vgs/jun03/vgs297.pdf . Vegetables and Melons Outlook/VGS-297. US Department of Agriculture ( ). Accessed 20 June 2003.
- 10.US Food and Drug Administration. http://www.fda.gov/bbs/topics/ANSWERS/2002/ ANS01167.html. http://www.fda.gov/bbs/topics/ANSWERS/2002/ ANS01167.html FDA issues import alert on cantaloupes from Mexico. US Food and Drug Administration. FDA Talk Paper. ). Accessed 28 October 2002.
- 11.US Food and Drug Administration. http://www.cfsan.fda.gov/~dms/prodsur6.html. http://www.cfsan.fda.gov/~dms/prodsur6.html FDA survey of imported fresh produce. FY 1999 Field Assignment. US Food and Drug Administration/Center for Food Safety and Applied Nutrition. ). Accessed 30 January 2003.
- 12.CDC. Aldicarb food poisoning from contaminated melons – California. Morb Mortal Wkly Rep. 1986;35:254–258. [PubMed] [Google Scholar]
- 13.Ries A, Zaza S, Langkop C Programs and abstracts of the 30th Interscience Conference on Antimicrobial Agents and Chemotherapy. Washington, DC: American Society for Microbiology; 1990. A multistate outbreak of Salmonella Chester linked to imported cantaloupe [Abstract] ; 1990. [Google Scholar]
- 14.CDC. Multistate outbreak of Salmonella poona infections – United States and Canada, 1991. Morb Mortal Wkly Rep. 1991;40:549–552. [PubMed] [Google Scholar]
- 15.del Rosario B, Beuchat L. Survival and growth of enterohemorrhagic Escherischia coli O157:H7 in cantaloupe and watermelon. J Food Prot. 1995;58:105–107. doi: 10.4315/0362-028X-58.1.105. [DOI] [PubMed] [Google Scholar]
- 16.Jackson L, Keene W, McAnulty J et al. Where’s the beef?: the role of cross-contamination in 4 chain restaurant-associated outbreaks of Escherichia coli O157:H7 in the Pacific Northwest. Arch Intern Med. 2000;160:2380–2385. doi: 10.1001/archinte.160.15.2380. [DOI] [PubMed] [Google Scholar]
- 17.Moehle-Boetani J, Reporter R, Werner S, Abbott S, Farrar J, Waterman S, Vugia D. An outbreak of Salmonella serogroup saphra due to cantaloupes from Mexico. J Inf Dis. 1999;180:1361–1364. doi: 10.1086/314995. [DOI] [PubMed] [Google Scholar]
- 18.Public Health Agency, Canada. Salmonella oranienburg, Ontario. Can Commun Dis Rep. 1998;24:177–179. [PubMed] [Google Scholar]
- 19.CDC. Multistate outbreaks of Salmonella serotype poona infections associated with eating cantaloupe from Mexico – United States and Canada, 2000–2002. Morb Mortal Wkly Rep. 2002;51:1044–1047. [PubMed] [Google Scholar]
- 20.Castillo A, Mercado I, Lucia L et al. Salmonella contamination during production of cantaloupe: a binational study. J Food Prot. 2004;67:713–720. doi: 10.4315/0362-028x-67.4.713. [DOI] [PubMed] [Google Scholar]
- 21.Richards G, Beuchat L. Attachment of Salmonella Poona to cantaloupe rind and stem scar tissues as affected by temperature of fruit and innoculum. J Food Prot. 2004;67:1359–1364. doi: 10.4315/0362-028x-67.7.1359. [DOI] [PubMed] [Google Scholar]
- 22.Suslow T, Cantwell M, Mitchell J. http://postharvest.ucdavis.edu/Produce/Producefacts/cantaloupe.html. http://postharvest.ucdavis.edu/Produce/Producefacts/cantaloupe.html Cantaloupes: Recommendations for Maintaining Postharvest Quality: Postharvest Technology Research and Information Center. University of California, Davis. ). Accessed 26 June 2004.
- 23.Richards G, Beuchat L. Metabiotic associations of molds and Salmonella Poona on intact and wounded cantaloupe rind. Int J Food Microbiol. 2005;97:327. doi: 10.1016/j.ijfoodmicro.2004.05.002. [DOI] [PubMed] [Google Scholar]
- 24.Richards G, Beuchat L. Infection of cantaloupe rind with Cladosporium cladosporioides and Penicillium expansum and associated migration of Salmonella Poona into edible tissues. Int J Food Microbiol. doi: 10.1016/j.ijfoodmicro.2004.05.023. (in press). [DOI] [PubMed] [Google Scholar]
- 25.Ukuku D, Fett W. Behavior of Listeria monocytogenes inoculated on cantaloupe surfaces and efficacy of washing treatments to reduce transfer from rind to fresh-cut pieces. J Food Prot. 2002;65:924–930. doi: 10.4315/0362-028x-65.6.924. [DOI] [PubMed] [Google Scholar]
- 26.Ukuku D, Sapers G. Effect of sanitizer treatments of Salmonella stanley attached to the surface of cantaloupe and cell transfer to fresh-cut tissues during cutting practices. J Food Prot. 2001;64:1286–1291. doi: 10.4315/0362-028x-64.9.1286. [DOI] [PubMed] [Google Scholar]
- 27.Ukuku D. Effect of hydrogen peroxide treatment on microbial quality and appearance of whole and fresh-cut melons contaminated with Salmonella species. Int J Food Microbiol. 2004;95:137–146. doi: 10.1016/j.ijfoodmicro.2004.01.021. [DOI] [PubMed] [Google Scholar]
- 28.Riordan D, Sapers G, Hankison T, Magee M, Mattrazzo A, Annous B. A study of US orchards to identify potential sources of Escherichia coli O157:H7. J Food Prot. 2001;64:1320–1327. doi: 10.4315/0362-028x-64.9.1320. [DOI] [PubMed] [Google Scholar]
- 29.Geldreich E, Bordner R. Fecal contamination of fruits and vegetables during cultivation and processing for market: a review. J Milk Food Technol. 1971;34:184–195. [Google Scholar]
- 30.Watkins J, Sleath K. Isolation and enumeration of Listeria monocytogenes from sewage, sewage sludge, and river water. J Appl Bacteriol. 1981;50:1–9. doi: 10.1111/j.1365-2672.1981.tb00865.x. [DOI] [PubMed] [Google Scholar]
- 31.Ingham S, Losinski J, Andrews M et al. Escherichia coli contamination of vegetables grown in soils fertilized with noncomposted bovine manure: garden-scale studies. Appl Environ Microbiol. 2004;70:6420–6427. doi: 10.1128/AEM.70.11.6420-6427.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Islam M, Morgan J, Doyle M, Phatak S, Millner P, Jiang X. Fate of avirulent Salmonella enterica serovar Typhimurium on selected vegetables grown in fields treated with contaminated manure composts or irrigation water. Appl Environ Microbiol. 2003;70:2497–2502. doi: 10.1128/AEM.70.4.2497-2502.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bagdasaryan G. Survival of viruses of the entero-virus group (poliomyelitis, echo, cocksakie) in soil and on vegetation. J Hyg Epid Microbiol Immun. 1964;8:497–505. [PubMed] [Google Scholar]
- 34.Mitscherlich E, Marth E. Microbial survival in the environment. New York: Springer-Verlag. 1984;173:372–376. : 350, [Google Scholar]
- 35.Caldwell K, Anderson G, Williams P, Beuchat L. Attraction of a free-living nematode, Caenorhabditis elegans, to foodborne pathogenic bacteria and its potential as a vector of Salmonella Poona for preharvest contamination of cantaloupe. J Food Prot. 2003;66:1964–1971. doi: 10.4315/0362-028x-66.11.1964. [DOI] [PubMed] [Google Scholar]
- 36.Brackett R. Shelf stability and safety of fresh produce as influenced by sanitation and disinfection. J Food Prot. 1992;55:808–814. doi: 10.4315/0362-028X-55.10.808. [DOI] [PubMed] [Google Scholar]
- 37.Gagliardi J, Millner P, Lester G, Ingram D. On-farm and postharvest processing sources of bacterial contamination to melon rinds. J Food Prot. 2003;66:82–87. doi: 10.4315/0362-028x-66.1.82. [DOI] [PubMed] [Google Scholar]
- 38.Solomon E, Yaron S, Matthews K. Transmission of Escherichia coli O157:H7 from contaminated manure and irrigation water to lettuce plant tissue and its subsequent internalization. Appl Environ Microbiol. 2002;68:397–400. doi: 10.1128/AEM.68.1.397-400.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Warriner K, Spaniolas S, Dickinson M, Wright C, Waites W. Internalization of bioluminescent Escherichia coli and Salmonella Montevideo in growing bean sprouts. J Appl Microbiol. 2003;95:719–727. doi: 10.1046/j.1365-2672.2003.02037.x. [DOI] [PubMed] [Google Scholar]
- 40.US Food and Drug Administration. www.cfsan.fda.gov/~lrd/tpproduc.html. www.cfsan.fda.gov/~lrd/tpproduc.html FDA advises consumers about fresh produce safety. US Food and Drug Administration. FDA Talk Paper. ). Accessed 26 May 2000.
- 41.Sapers G, Miller R, Pilizota V, Mattrazzo A. Antimicrobial treatments for minimally processed cantaloupe melon. J Food Sci. 2001;66:345–349. [Google Scholar]
- 42.Wade W, Beuchat L. Metabiosis of proteolytic moulds and Salmonella in raw, ripe tomatoes. J Appl Microbiol. 2003;95:437–450. doi: 10.1046/j.1365-2672.2003.01995.x. [DOI] [PubMed] [Google Scholar]
- 43.Burnett S, Chen J, Beuchat L. Attachment of Escherichia coli O157:H7 to the surface and internal structures of apples as demonstrated by confocal scanning laser microscopy. Appl Environ Microbiol. 2000;66:4679–4687. doi: 10.1128/aem.66.11.4679-4687.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Golden D, Rhodehamel E, Kautter D. Growth of Salmonella spp. in cantaloupe, watermelon and honeydew melons. J Food Prot. 1993;56:194–196. doi: 10.4315/0362-028X-56.3.194. [DOI] [PubMed] [Google Scholar]
- 45.Beuchat L, Scouten A. Factors affecting survival, growth, and retrieval of Salmonella Poona on intact and wounded cantaloupe rind and in stem scar tissue. Food Microbiol. 2004;21:683–694. [Google Scholar]
- 46.Richards G, Buck J, Beuchat L. Survey of yeasts for antagonistic activity against Salmonella Poona in cantaloupe juice and wounds in rinds co-infected with phytopathogenic molds. J Food Prot. 2004;67:2132–2142. doi: 10.4315/0362-028x-67.10.2132. [DOI] [PubMed] [Google Scholar]
- 47.Ukuku D, Fett W. Relationship of cell surface charge and hydrophobicity to strength of attachment of bacteria to cantaloupe rind. J Food Prot. 2002;65:1093–1099. doi: 10.4315/0362-028x-65.7.1093. [DOI] [PubMed] [Google Scholar]
- 48.Leverentz B, Conway W, Alavidze Z, Janisiewicz W et al. Examination of bacteriophage as a biocontrol method for Salmonella on fresh-cut fruit: a model study. J Food Prot. 2001;64:1116–1121. doi: 10.4315/0362-028x-64.8.1116. [DOI] [PubMed] [Google Scholar]
- 49.Escartin E, Ayala A, Lozano J. Survival and growth of Salmonella and Shigella on sliced fresh fruit. J Food Prot. 1989;52:471–472. doi: 10.4315/0362-028X-52.7.471. [DOI] [PubMed] [Google Scholar]
- 50.Penteado A, Leitao M. Growth of Listeria monocytogenes in melon, watermelon, and papaya pulps. Int J Food Microbiol. 2004;92:89–94. doi: 10.1016/j.ijfoodmicro.2003.08.020. [DOI] [PubMed] [Google Scholar]
- 51.Ukuku D, Pilizota V, Sapers G. Effect of hot water and hydrogen peroxide treatments on survival of Salmonella and microbial quality of whole and fresh-cut cantaloupe. J Food Prot. 2004;67:432–437. doi: 10.4315/0362-028x-67.3.432. [DOI] [PubMed] [Google Scholar]
- 52.Lin C, Wei C. Transfer of Salmonella montevideo on to the interior surfaces of tomatoes by cutting. J Food Prot. 1997;60:858–863. doi: 10.4315/0362-028X-60.7.858. [DOI] [PubMed] [Google Scholar]
- 53.Laminkanra O, Chen J, Banks D, Hunter P. Biochemical and microbiological changes during the storage of minimally processed cantaloupe. J Agric Food Chem. 2000;48:5955–5961. doi: 10.1021/jf0000732. [DOI] [PubMed] [Google Scholar]
- 54.Barak J, Chue B, Mills D. Recovery of surface bacteria from and surface sanitation of cantaloupes. J Food Prot. 2003;66:1805–1810. doi: 10.4315/0362-028x-66.10.1805. [DOI] [PubMed] [Google Scholar]
- 55.Annous B, Burke A, Stiles J. Surface pasteurization of whole fresh cantaloupes inoculated with Salmonella Poona or Escherichia coli. J Food Prot. 2004;67:1876–1885. doi: 10.4315/0362-028x-67.9.1876. [DOI] [PubMed] [Google Scholar]
- 56.Ukuku D, Fett W. Method of applying sanitizers and sample preparation affects recovery of native microflora and Salmonella on whole cantaloupe surfaces. J Food Prot. 2004;67:999–1004. doi: 10.4315/0362-028x-67.5.999. [DOI] [PubMed] [Google Scholar]
- 57.Sapers G, Stiles J. Efficacy of 1% hydrogen peroxide wash in decontaminating apples and cantaloupe melons. J Food Sci. 2003;68:1793–1797. [Google Scholar]
- 58.Ukuku D, Fett W. Effect of nisin in combination with EDTA, sodium lactate, and potassium sorbate for reducing Salmonella on whole and fresh-cut cantaloupe. J Food Prot. 2004;67:2143–2150. doi: 10.4315/0362-028x-67.10.2143. [DOI] [PubMed] [Google Scholar]
- 59.Monk J, Beuchat L, Doyle M. Irradiation inactivation of food-borne microorganisms. J Food Prot. 1995;58:197–208. doi: 10.4315/0362-028X-58.2.197. [DOI] [PubMed] [Google Scholar]
- 60.Larson A, Johnson E. Evaluation of botulinum toxin production in packaged fresh-cut cantaloupe and honeydew melon. J Food Prot. 1999;62:948–952. doi: 10.4315/0362-028x-62.8.948. [DOI] [PubMed] [Google Scholar]
- 61.Wright J, Sumner S, Hackney C, Pierson M, Zoecklein B. Efficacy of ultraviolet light for reducing Escherichia coli O157:H7 in unpasteurized apple cider. J Food Prot. 2000;63:563–567. doi: 10.4315/0362-028x-63.5.563. [DOI] [PubMed] [Google Scholar]
- 62.Ukuku D, Pilizota V, Sapers G. Bioluminescence ATP assay for estimating total plate counts of surface microflora of whole cantaloupe and determining efficacy of washing treatments. J Food Prot. 2001;64:813–819. doi: 10.4315/0362-028x-64.6.813. [DOI] [PubMed] [Google Scholar]
- 63.Carlin F, Nguyen-The C, Morris C. Influence of background microflora on Listeria monocytogenes on minimally processed fresh broad-leaved endive. J Food Prot. 1996;59:698–703. doi: 10.4315/0362-028X-59.7.698. [DOI] [PubMed] [Google Scholar]
- 64.Mead P, Slutsker L, Dietz V et al. Food-related illness and death in the United States. Emerg Infect Dis. 1999;5:607–625. doi: 10.3201/eid0505.990502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.US Food and Drug Administration. http://www.cfsan.gov/~dms/prodguid.html. 2003. http://www.cfsan.gov/~dms/prodguid.html Guidance for Industry. Guide to minimize microbial food safety hazards for fresh fruits and vegetables. US Food and Drug Administration/ Center for Food Safety and Applied Nutrition. ). Accessed 26 October.
- 66.US Food and Drug Administration. http://vm.cfsan.fda.gov/~ear/ret-mln.html. http://vm.cfsan.fda.gov/~ear/ret-mln.html Produce safety at retail: safe handling practices for melons. US Food and Drug Administration/Center for Food Safety and Applied Nutrition. ). Accessed 25 May 2004.
- 67.Suslow T. Overview of industry practices. Minimizing the risk of foodborne illness associated with cantaloupe production and handling in California. Davis, California: University of California; 2004. [7 January 2005]. ; 24 pp ( [Google Scholar]
- 68.Rangarajan A, Bihn E, Gravani R, Scott D, Pritts M. Food Safety Begins on the Farm. Ithaca, NY: Cornell University; 2004. pp. 1–28. [Google Scholar]
- 69.Gorny JR. Food safety guidelines for the fresh-cut produce industry. 4th edn. Alexandria, VA: International Fresh-cut Produce Association; 2001. pp. 1–219. [Google Scholar]
- 70.Garrett E, McInerney M, Hempel J. Voluntary food safety guidelines for fresh produce: international fresh-cut produce association, Alexandria, VA and Western Growers Association. Irvine, CA: 1997. [Google Scholar]
- 71.US Food and Drug Administration. http://www.cfsan.fda.gov/~dms/prodsu10.html. http://www.cfsan.fda.gov/~dms/prodsu10.html FDA survey of domestic fresh produce. US Food and Drug Administration/Center for Food Safety and Applied Nutrition. ). Accessed January 2003.