SUMMARY
To investigate risk factors during a community outbreak of hepatitis A we carried out a case- control study of 35 cases and 49 matched controls using an interviewer-administered questionnaire on clinical history, travel, household details including domestic toilet facilities, infectious contacts, and food history. Of 99 cases notified in the city during the outbreak year, 50 (51%) were young adults age 15–34 years. Hepatitis A infection was independently associated with household contact with a case (P=0·0005/xref>), and sharing a household with children in primary school (OR 3·4, 95% CI 1·2–9·5, P=0·008) with risk increasing with number of primary-school pupils in the household (χ2 for linear trend 6·47, P=0·01). We concluded that in a population with a low prevalence of hepatitis A, adults who live in the same household as primary-school-age children are at increased risk of acquiring the infection during community outbreaks.
INTRODUCTION
Hepatitis A infection is caused by the HAV picornavirus and is endemic worldwide. Following an incubation period of 2–8 weeks, symptoms include fever, malaise, loss of appetite, nausea and abdominal discomfort, followed by jaundice. Disease is usually mild and lasts 1–2 weeks, but may be severe and last for several months. It is usually asymptomatic in young children, but the likelihood of symptomatic disease, morbidity and mortality increases with age, with a case-fatality rate of 1·5% in those aged >64 years in the United Kingdom from 1980 to 1989 [1].
Man is the reservoir of HAV, which is spread by the faecal–oral route. The virus is most commonly transmitted by direct person-to-person contact, but sometimes by contaminated food and water. Identified risk factors for infection in the United Kingdom include household contact with another diagnosed case, travel abroad, sharing a household with a child aged 3–10 years, and consumption of shellfish harvested from sewage-polluted waters [2, 3]. Outbreaks occur in nurseries [4], infant schools [5] and institutions for the mentally handicapped [6, 7], among intravenous drug users [8–11], men who have sex with men [12–16], and rarely as a result of food contamination [1, 17–19] and public swimming-pool use [20].
Notifications of hepatitis A in the United Kingdom (originally as infective jaundice) fell from 23 580 in 1969 to 3216 in 1979, and incidence has remained low [1, 21]. The number of laboratory reports of hepatitis A in England and Wales has declined every year since 1991, with the exception of 2002, but the trend resumed in 2003 when there were 984 laboratory reports and 1194 notifications [22]. Therefore, the proportion of susceptible adults is increasing, and studies suggest that most children and young adults in the United Kingdom are now susceptible to hepatitis A infection, as is increasingly the case in Northern Europe [1, 23, 24].
We report a case-control study to investigate risk factors for infection and exclude a point source of infection during an investigation of a community-wide outbreak of hepatitis A mainly affecting young adults in Swansea, Wales.
The outbreak
A total of 99 cases of hepatitis A (53 male and 46 female) were notified among residents of Swansea (population 182 000) between January and December, an attack rate of 54/100 000 per year. Thirty-five (35%) were <15 years, 50 (51%) were age 15–34 years, and 14 (14%) were >34 years. Serological confirmation was reported for 74 cases. No deaths occurred, but 12 cases were admitted to hospital.
A significant proportion of cases (40%) were resident in the city’s most socio-economically deprived ward, which had an annual incidence of 35/10 000, compared to 3·6/10 000 in the rest of the city. The annual incidence among young adults (age 15–34 years) in this ward was 80/10 000.
During the outbreak environmental health officers visited primary schools in the city to investigate pupils’ toilet facilities. Out of 10 school visited, only two had satisfactory facilities. Problems included no hand washbasin adjacent to the toilets (five), no soap (four), no hot water (four), no toilet paper (two) and no toilet seats in the girls’ toilets (one).
METHOD
We conducted a case-control study of notified cases. Initially two controls per case were sought from the same general practitioner’s list, matched by sex and 5-year age band. However, due to limited resources, the target number of controls was reduced to one per case mid-way through the study. A case was defined as any resident who had a clinical or serological diagnosis of hepatitis A, with an onset of jaundice, or clinical illness, in the last 6 months of the outbreak year. Case finding was conducted by contacting all local general practitioners and known cases.
Data on demographic details, clinical history, travel, household details including the presence of hand washbasins in domestic toilets, infectious contacts and food history in the 2 months before the onset of jaundice were collected by questionnaire administered by an interviewer following a standard interview procedure. An infectious contact was assumed to have been the source of infection for a case if the onset of jaundice in the case occurred from 1 week before to 7 weeks after the onset of jaundice in the contact [25]. Subjects were randomly assigned one of three interviewers, and where possible interviews were conducted by telephone.
Odds ratios were calculated for 2×2 tables using unmatched analysis, and significance tested using Mantel–Haenszel χ2 test, with 95% confidence limits derived using Cornfield’s approximation. Fisher’s exact test was used where an expected cell value was <5. Stratified analysis was used to indicate whether or not associations on single table unmatched analysis were due to confounding. A deprived area was defined as an electoral ward having a Townsend index >6 [26]. Data were analysed using Epi-Info software [27].
RESULTS
Questionnaires were completed for 35 of the 45 cases and 49 of the 53 controls, a response rate of 78% for cases and 92% for controls (86% overall). Case finding suggested that notification was largely complete. Cases consisted of two pre-school children, seven primary-school pupils, five secondary-school pupils and 21 adults, and were similar to all cases notified that year with regard to sex, age and residential district.
Risk factor analysis
In unmatched univariate analysis (Table 1) infection with hepatitis A was associated with household contact with an infectious case (OR undefined, P=0·0005), sharing a household with children in primary school (OR 3·4, P=0·008), and stratified analysis showed these associations were independent. Cases were more likely to live in a deprived area (OR 2·6, P=0·04). No association was found with any other risk factors indicated in Table 1, or any putative point source.
Table 1.
Results of unmatched analyses for selected risk factors
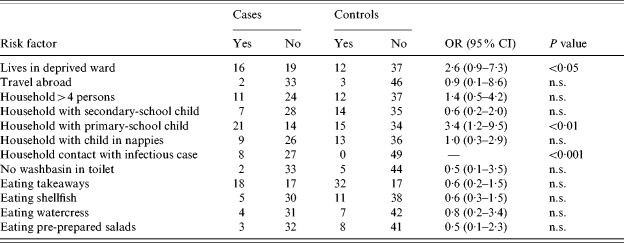
OR, Odds ratio; CI, confidence interval; n.s., not significant.
Eight of the 35 cases shared a household with one or more hepatitis A cases during a period when they could have been infected. Of the remaining 27 cases, 17 (63%) lived in a household where there were children in primary school, compared with 15 controls (31%) (OR 3·8, 95% CI 1·3–11·8, P=0·007). Analysis for linear trend in proportions of cases and controls who lived with zero, one or two primary-school pupils (Table 2) showed a significant trend both when all cases were included (P=0·011), and when the 27 cases who did not report close contact with an infectious case were analysed separately (P=0·0062).
Table 2.
Analysis of linear trend in proportions of primary-school pupils in the household of cases and controls
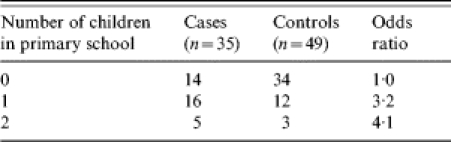
for linear trend 6·47, P=0·011.
Morbidity of cases
The average length of self-defined illness among cases was 20 days, and ranged from 3 to 56 days, the mode was 7–14 days, and the mean number of general practitioner consultations was 2·9. Average length of stay among 10 cases admitted to hospital was 3 days in children (n=3) and 5·6 days in adults (n=7), range 1–7 days.
DISCUSSION
We found hepatitis A infection was associated with sharing a household with a child in primary school, with risk increasing as the number of primary-school pupils sharing the household increased.
An association with children attending day-care centres has previously been suggested [28]. Two other studies have demonstrated an increased risk of hepatitis A associated with contact with young children, one a large case-control study of sporadic cases in the United Kingdom [3], the other a case control of a community outbreak in the United States [29]. That study was conducted in Florida, where 311 cases occurred over 13 months in three cities. A matched ‘household’ case-control study was conducted which showed significant independent associations between having a case of hepatitis A in the household and three variables: child cared for in a day-care centre, low educational status, and contact with a hepatitis A case outside the household. HAV is spread from pupil to pupil during outbreaks, and a significantly increased risk of illness among those using the same toilet as a case has been demonstrated [4, 5]. There is evidence that our experience of inadequate toilet facilities in school is common in the United Kingdom [30].
Outbreaks of hepatitis A are difficult to study because of the high proportion of asymptomatic cases, and the long incubation makes recall of exposures difficult [31]. Some of the cases and controls interviewed were asked to recall details between 1 and 9 months after the event. Many of the variables of interest, in particular sharing a household with a case or primary-school children, were likely to have been recalled accurately, although data on food history may be less reliable. Selection bias was minimized by the good response rate, and interviewers used a standard questionnaire and followed a standard format to minimize interviewer-associated biases. Distribution by sex, age group and district of residence among study cases was similar to all notified cases that year.
The association between hepatitis A and sharing a household with a primary-school child was a prior hypothesis, and not a finding generated by testing multiple associations. A causal association is biologically plausible, because an asymptomatic infected child may expose others in the household to the virus, which then causes a symptomatic infection in an adult or another child. The association we found is, therefore, likely to be of public health importance.
Although advising household contacts of children in primary school that they are at increased risk of hepatitis A infection during community outbreaks is a reasonable precaution, preventing infection in young children though prophylactic immunization raises difficult ethical issues [32]. Although immunization of young children is likely to reduce the risk to other members of the household, it also prevents mild or asymptomatic infection in the immunized child, which would produce long-lasting immunity in that individual. Conversely, the protection provided by a single dose of vaccine would wane before the child entered adult life, leaving that individual susceptible at an age when complications from hepatitis A are more likely. Therefore, while immunization may benefit the household, there may be disbenefits for the individual child, and the decision of whether or not to immunize young children during outbreaks is challenging. This is compounded by a lack of data on severity of illness in children admitted to hospital with hepatitis A, and further research in this area may help define at what age the individual short-term benefits of immunization outweigh the long-term benefits of natural infection with HAV at an early age.
ACKNOWLEDGEMENTS
We are grateful to many individual members of the Communicable Disease Surveillance Centre (Wales), West Glamorgan Department of Public Health Medicine and Swansea City Council Environmental Health Services Department for their help in carrying out this investigation.
DECLARATION OF INTEREST
None.
REFERENCES
- 1.Tilzey AJ, Banatvala JE. Hepatitis A. BMJ. 1991;302:1552–1553. doi: 10.1136/bmj.302.6792.1552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Maguire H, Heptonstall J, Begg NT. The epidemiology and control of hepatitis A. Communicable Disease Report. 1992;2:R114–R117. [PubMed] [Google Scholar]
- 3.Maguire HC et al. A collaborative case control study of sporadic hepatitis A in England. Communicable Disease Report. 1995;5:R33–R40. [PubMed] [Google Scholar]
- 4.Joseph C et al. A review of outbreaks of infectious disease in schools in England and Wales 1979–88. Epidemiology and Infection. 1990;105:419–434. doi: 10.1017/s0950268800048007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rajaratnam G et al. An outbreak of hepatitis A: school toilets as a source of transmission. Journal of Public Health Medicine. 1992;14:72–77. [PubMed] [Google Scholar]
- 6.Szmuness W et al. Antibody to hepatitis A antigen in institutionalized mentally retarded patients. Journal of the American Medical Association. 1977;237:1702–1705. [PubMed] [Google Scholar]
- 7.Ang LH. Outbreak of hepatitis A in a special needs school in Kent: 1999. Communicable Disease and Public Health. 2000;3:139–140. [PubMed] [Google Scholar]
- 8.Harkess J, Gildon B, Istre GR. Outbreaks of hepatitis A among illicit drug users, Oklahoma, 1984–87. American Journal of Public Health. 1989;79:463–466. doi: 10.2105/ajph.79.4.463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hutin YJ et al. Identifying target groups for a potential vaccination program during a hepatitis A communitywide outbreak [published Erratum appears in American Journal of Public Health 1999; 89: 1274] American Journal of Public Health. 1999;89:918–921. doi: 10.2105/ajph.89.6.918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shaw DD et al. Hepatitis A outbreaks among illicit drug users and their contacts in Queensland, 1997. Medical Journal of Australia. 1999;170:584–587. doi: 10.5694/j.1326-5377.1999.tb127904.x. [DOI] [PubMed] [Google Scholar]
- 11.Hutin YJ et al. Multiple modes of hepatitis A virus transmission among methamphetamine users. American Journal of Epidemiology. 2000;152:186–192. doi: 10.1093/aje/152.2.186. [DOI] [PubMed] [Google Scholar]
- 12.Corey L, Holmes KK. Sexual transmission of hepatitis A in homosexual men: incidence and mechanism. New England Journal of Medicine. 1980;302:435–438. doi: 10.1056/NEJM198002213020804. [DOI] [PubMed] [Google Scholar]
- 13.Anon. Hepatitis A among homosexual men – United States, Canada and Australia. Morbidity and Mortality Weekly Report. 1992;41:155–164. [PubMed] [Google Scholar]
- 14.Coutinho RA et al. Prevalence and incidence of hepatitis A among male homosexuals. BMJ (Clinical Research Edition) 1983;287:1743–1745. doi: 10.1136/bmj.287.6407.1743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Henning KJ et al. A community-wide outbreak of hepatitis A: risk factors for infection among homosexual and bisexual men. American Journal of Medicine. 1995;99:132–136. doi: 10.1016/s0002-9343(99)80132-6. [DOI] [PubMed] [Google Scholar]
- 16.Communicable Disease Surveillance Centre. Hepatitis A in homosexual men. Communicable Disease Report CDR Weekly. 1996;6:247. [PubMed] [Google Scholar]
- 17.Nui MT et al. Multistate outbreak of hepatitis A associated with frozen strawberries. Journal of Infectious Diseases. 1992;166:518–524. doi: 10.1093/infdis/166.3.518. [DOI] [PubMed] [Google Scholar]
- 18.Rosenblum LS et al. A multifocal outbreak of hepatitis A traced to commercially distributed lettuce. American Journal of Public Health. 1990;80:1075–1079. doi: 10.2105/ajph.80.9.1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Skala M et al. Foodborne hepatitis A – Missouri, Wisconsin, and Alaska, 1990–1992. Morbidity and Mortality Weekly Report. 1993;42:526–529. [PubMed] [Google Scholar]
- 20.Mahoney FJ et al. An outbreak of hepatitis A associated with swimming in a public pool. Journal of Infectious Diseases. 1992;165:613–618. doi: 10.1093/infdis/165.4.613. [DOI] [PubMed] [Google Scholar]
- 21.Office of Population Censuses and Surveys. London: OPCS; 1993. C91, 1991 Census data for personal computers. [Google Scholar]
- 22.Health Protection Agency Communicable Disease Surveillance Centre. Laboratory reports of hepatitis A in England and Wales; 2003. http://www.hpa.org.uk/cdr Communicable Disease Report CDR Weekly. 2004;14(35) [serial online] [cited 7 September 2004]; ) (available at: [Google Scholar]
- 23.Gay NJ et al. Age-specific antibody prevalence to hepatitis A in England: implications for disease control. Epidemiology and Infection. 1994;113:113–120. doi: 10.1017/s0950268800051529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Melnick JL. History and epidemiology of hepatitis A virus. Journal of Infectious Diseases. 1995;171:S2–S8. doi: 10.1093/infdis/171.supplement_1.s2. (Suppl 1): [DOI] [PubMed] [Google Scholar]
- 25.Weatherall DJ, Ledingham JGG, Warrell DA. Oxford Textbook of Medicine. 2nd edn. Oxford: Oxford University Press; 1987. [Google Scholar]
- 26.Townsend P, Phillimore P, Beattie A. Health and Deprivation: inequality and the North. London: Croom Helm; 1988. [Google Scholar]
- 27.Dean AD Epi-Info, Version 5: a word processing, database, and statistics program for epidemiology on micro-computers. Stone Mountain, GA: USD Inc.; 1990. [Google Scholar]
- 28.Hadler SC et al. Hepatitis A in day-care centres: a community-wide assessment. New England Journal of Medicine. 1980;302:1222–1227. doi: 10.1056/NEJM198005293022203. [DOI] [PubMed] [Google Scholar]
- 29.Desenclos JA, Maclafferty L. Community wide outbreak of hepatitis A linked to children in day care centres and with increased transmission in young adult men in Florida 1988–9. Journal of Epidemiology and Community Health. 1993;47:269–273. doi: 10.1136/jech.47.4.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jewkes RK, O’Connor BH. Crisis in our schools: survey of sanitation facilities in schools in Bloomsbury health district. BMJ. 1990;301:1085–1087. doi: 10.1136/bmj.301.6760.1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Joseph CA et al. An outbreak of hepatitis A. Communicable Disease Report. 1992;2:R17–R18. [PubMed] [Google Scholar]
- 32.Crowcroft NS et al. Guidelines for the control of hepatitis A virus infection. Communicable Disease and Public Health. 2001;4:213–227. [PubMed] [Google Scholar]


