SUMMARY
Enteric illness outbreaks among middle-/high-school students in consecutive semesters of an educational farm programme were investigated with retrospective cohort studies. During the first outbreak, 31/92 (34%) interviewed students were ill. Risk factors included participating in animal science class (RR 8·1, 95% CI 1·2–55·2) and contact with calves (RR 4·2, 95% CI 1·1–16·2). Stool samples from seven students and two calves yielded Cryptosporidium parvum. Students cared for animals in street clothes and practised poor hand washing. During the second outbreak, 37/81 (46%) interviewed animal science students were ill. Risk factors included having visible manure on hands, and wearing coveralls and boots. Stool samples from seven students and eight calves yielded C. parvum. Student hand washing was still inadequate. Coveralls/boots were cleaned infrequently and removed after hand washing. These outbreaks of cryptosporidiosis resulted from calf contact and inadequate hygiene practices. The failure to adequately implement recommended interventions contributed to the second outbreak.
INTRODUCTION
Cryptosporidiosis is a frequent cause of diarrhoeal disease among humans and animals caused by Cryptosporidium, a protozoan parasite. C. parvum was recognized as an enteric pathogen of cattle in 1971 [1]. Human cryptosporidiosis was first identified in 1976, but was reported only sporadically until 1982 [2, 3], when the number of recognized cases increased dramatically among immunocompromised persons associated with the HIV/AIDS epidemic [4]. More recently, Cryptosporidium has been recognized as an important pathogen among immunocompetent hosts as well [2–5].
Two main species of Cryptosporidium cause human illness: C. hominis (formerly known as C. parvum genotype 1), which has a human reservoir and infects only humans; and C. parvum (formerly known as C. parvum genotype 2), which infects primarily ruminants and humans [6]. Among humans, waterborne transmission is the most commonly reported mode of transmission of Cryptosporidium oocysts; however, person-to-person and foodborne spread also occur. Zoonotic transmission of C. parvum through direct contact with ruminant animals to immunocompetent human hosts was first reported in 1983 [2, 3]. Since that time, numerous outbreaks of cryptosporidiosis have been documented among students at veterinary colleges [7–9]. More recently, a broader public health threat in the form of cryptosporidiosis outbreaks at venues where the public contacts farm animals (e.g. petting zoos or educational farms) has been recognized [10, 11]. However, reports of such outbreaks have been rare in the United States.
In this report, we summarize the epidemiological, environmental, and laboratory investigations of two outbreaks of cryptosporidiosis among middle- and high-school students in consecutive semesters of an educational farm programme administered by a northern Minnesota school district.
METHODS
In February 2003 and again in September 2003, the Minnesota Department of Health (MDH) was notified of multiple students who were ill with cryptosporidiosis and who all attended the same educational farm programme administered by the local school district. During those months, no cryptosporidiosis cases unrelated to the farm programme were reported to MDH from the county in which the school district was located. Interviews with the school principals and programme instructors were conducted to obtain an overview of the programme. A cohort study, environmental investigation, and laboratory stool testing of students and animals were conducted for each outbreak.
For the cohort studies, students were interviewed by telephone to obtain illness and exposure histories, including specific animal contact activities, hand-hygiene practices, use of protective clothing, and eating/drinking on the premises. During the first outbreak, the cohort consisted of students who attended class in the barn-classroom complex (i.e. either animal science, equine science, or greenhouse class) to ascertain whether participation in any one class or other exposures related to the complex (e.g. drinking water) were associated with illness. Based on the results of the first outbreak investigation and the observation that the index cases in the second outbreak attended the animal science class but not other farm classes, the cohort during the second outbreak investigation was limited to students from the animal science class only.
Case definitions
Two case definitions were employed, and analyses were conducted separately using each case definition. In case definition 1, a case was defined as vomiting or diarrhoea (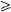 3 loose stools in 24 h) occurring in a student after the first day of the semester. In analyses using case definition 1, students reporting mild gastrointestinal illness symptoms that did not meet this case definition were excluded from analyses. A more sensitive case definition (case definition 2) was also evaluated to see if inferences would have changed by including mild illnesses as cases; in this definition a case was defined as any gastrointestinal illness symptom (e.g. any loose stools, abdominal cramps, or nausea) occurring in a student after the first day of the semester.
3 loose stools in 24 h) occurring in a student after the first day of the semester. In analyses using case definition 1, students reporting mild gastrointestinal illness symptoms that did not meet this case definition were excluded from analyses. A more sensitive case definition (case definition 2) was also evaluated to see if inferences would have changed by including mild illnesses as cases; in this definition a case was defined as any gastrointestinal illness symptom (e.g. any loose stools, abdominal cramps, or nausea) occurring in a student after the first day of the semester.
Environmental investigations were conducted during each outbreak to observe students during class, to obtain purchase and illness histories of the animals, and to collect faecal specimens from the animals.
Stool testing was performed on stools from both ill students and from animals with which the students had contact. Animal faecal samples were obtained rectally and stored in ova and parasite medium [10% formalin and mercuric chloride (Meridian Bioscience Inc., Cincinnati, OH, USA)] and Cary–Blair bacterial transport medium. Stool kits containing the same two transport media were mailed to the students, self-collected, then returned by mail. The MDH Public Health Laboratory (PHL) conducted routine cultures for enteric bacteria, routine ova and parasite examination, and additional tests for Cryptosporidium and Giardia [acid-fast staining and direct fluorescent antibody tests (Merifluor, Meridian Bioscience Inc.)] [12]. Polymerase chain reaction (PCR) for Cryptosporidium was performed on all stool samples, and all Cryptosporidium amplification products were identified to species using restriction fragment length polymorphism (PCR–RFLP) [13]. Stool samples from infected index case-patients were submitted from the regional hospital laboratory to the MDH PHL for Cryptosporidium PCR–RFLP. PCR testing for the presence of Shiga toxin genes (stx1 and stx2) was performed on ‘sweeps’ of bacterial colonies from sorbitol MacConkey plates [14]. Escherichia coli isolates that were positive for Shiga toxin genes were serotyped using antisera agglutination [E. coli diagnostic antisera (Statens Serum Institut, Copenhagen, Denmark, or Denka Seiken Co. Ltd, Tokyo, Japan)] then sent to the Centers for Disease Control and Prevention (CDC) for confirmation.
Univariate analyses by Pearson uncorrected χ2 test were performed using Epi-Info software, version 6.04 (CDC, Atlanta, GA, USA). Exact methods were used if cell sizes were insufficient. Variables with a P value of  0·10 in univariate analysis were entered into a multivariate stepwise logistic regression model. Multivariate analysis was performed using SAS System for Windows, version 8.2 (SAS Institute Inc., Cary, NC, USA). In the multivariate analysis, adjusted odds ratios were reported to provide an estimate of risk ratios (RRs).
0·10 in univariate analysis were entered into a multivariate stepwise logistic regression model. Multivariate analysis was performed using SAS System for Windows, version 8.2 (SAS Institute Inc., Cary, NC, USA). In the multivariate analysis, adjusted odds ratios were reported to provide an estimate of risk ratios (RRs).
RESULTS
The farm programme consisted of classes in animal science, equine science, greenhouse, small engines, pet science, power mechanics, and natural resources. Participants (9th–12th graders from the high school and two middle schools in the district), were transported to the farm campus for classes. Classes lasted for 1 h, Monday–Friday. Students in the animal science class had direct contact with calves, horses, goats, sheep, and rabbits. Students in the equine science class had direct contact with horses only. Animal science students were responsible for feeding, grooming, haltering, and leading calves, as well as cleaning out calf pens.
First outbreak, February 2003
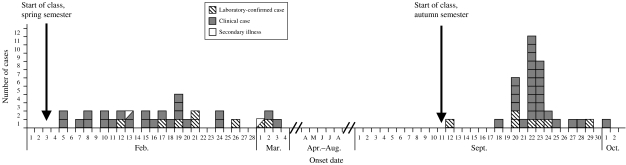
During the spring semester (first outbreak), 101 students were enrolled in the animal science, equine science, or greenhouse classes. Ninety-two (91%) students were interviewed, and 31 (34%) met case definition 1. In addition, eight students reported mild gastrointestinal illness symptoms that did not meet the case definition 1; these students were excluded from the first analysis. Illness onsets for case-patients ranged from 2 days to 1 month after the beginning of the semester (Fig.). Case-patients were distributed among all three schools and all four grades; no differences in attack rates by school or grade were found. Two secondary illness cases were identified, both of whom were siblings of case-patients who were students in the animal science class. Among the 31 student case-patients, the median duration of illness was 7 days (range 6 h to 19 days). Symptoms included diarrhoea (90%), abdominal cramps (66%), vomiting (43%), and fever (17%). One student required hospitalization for 2 days. Of the 31 ill students, nine visited a health-care provider, and two had stool samples submitted for enteric pathogen testing by their providers.
Fig.
Cryptosporidiosis cases by date of illness onset, spring and autumn semesters, 2003. Date of illness onset was available for 30 students during spring semester and 35 students during autumn semester.
Stool samples were received at the MDH PHL from eight ill students and the two ill siblings (secondary cases). Cryptosporidium was identified in the specimens from seven students and one ill sibling; all eight positives were confirmed as C. parvum by PCR–RFLP. All specimens were negative for Giardia, Campylobacter, Salmonella, E. coli O157:H7, and Shiga toxin genes.
In the cohort study using case definition 1, participating in the animal science class was associated with illness, with an attack rate of 48% among animal science students vs. 6% of non-animal science students, yielding a RR of 8·1 (Table 1). Contact with calves was also associated with illness (RR 4·2) (Table 1). Contact with other animal species was not associated with illness.
Table 1.
Potential risk factors for cryptosporidiosis among students in the animal science, equine science, and greenhouse classes during the first outbreak, Minnesota, February 2003a
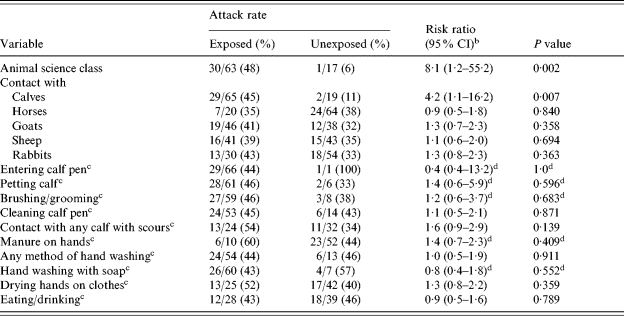
Students enrolled in more than one of the three classes were excluded from evaluation of each class as a potential risk factor. Students reporting mild gastrointestinal illness symptoms that did not meet the case definition (i.e. vomiting or 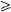 3 loose stools in 24 h) were excluded from analyses shown in this table.
3 loose stools in 24 h) were excluded from analyses shown in this table.
CI, Confidence interval.
Among animal science students only.
Represents exact confidence interval and exact P value.
Among animal science class students only, specific calf-related activities (e.g. entering a calf pen, petting or grooming a calf, cleaning a pen, caring for a calf with scours, and getting visible manure on one’s hands) were common exposures (Table 1), but none were significantly associated with illness. No individual calf was associated with illness. Other specific hygiene practices (e.g. lack of hand washing, lack of use of soap, drying hands on one’s clothing, or eating or drinking while on the farm campus), although prevalent, were not statistically associated with illness (Table 1).
In the analysis using case definition 2, findings were the same as for case definition 1; participating in animal science class and contact with calves were the only two variables associated with illness (data not shown).
The environmental investigation revealed that animal science students routinely cared for animals in their street clothes and shoes. Coveralls and boots were available but were not mandatory. Students were given approximately 3–5 min at the end of class to wash before returning to school. We observed 20–25 students clustered around two sinks in the barn, performing cursory, inadequate hand washing. Students scrubbed their shoes with shared dry brushes.
Ten 3-day-old calves were purchased from a sales barn 2 days prior to the start of the semester. Upon arrival, several calves were scouring and one died within days. Another calf was seen by the attending veterinarian and was diagnosed with cryptosporidiosis. At the time of the site visit, the calves were approximately 6 weeks old and appeared healthy (along with the rest of the animals at the farm facility). Of the nine remaining calves, eight tested positive for at least one pathogen: Giardia (n=3), Campylobacter coli (n=2), C. jejuni (n=1), Giardia and Cryptosporidium (n=1), and Giardia and C. coli (n=1). The Cryptosporidium-positive sample did not amplify by PCR–RFLP. A gene for Shiga toxin (stx1) was detected in bacteria cultured from two calves. One calf was positive for E. coli O121, and one was positive for E. coli O111.
After completion of the outbreak investigation, MDH recommended a series of interventions for the agricultural programme. These included prohibiting food and drink on the premises, assigning students to one of six sinks located throughout the building, implementing supervised hand washing, placing alcohol-based sanitizer gel at the building exit, and having students use rubber boots and coveralls when working with calves. The farm manager was encouraged to purchase calves from a single source, to isolate ill calves, and to prevent student interaction with ill calves.
Second outbreak, September 2003
Seven months later, at the beginning of the autumn semester, a second outbreak of cryptosporidiosis occurred among participants of the same programme (Fig.). The investigation of this recurrence focused on students in the animal science class and an apparent failure to implement the previous recommendations. Ninety-one students were enrolled in the animal science class; 81 (89%) were interviewed, and 37 (46%) of those met case definition 1. In addition, four students reported mild gastrointestinal illness symptoms that did not meet case definition 1; these students were excluded from the first analysis. Among the 37 student case-patients, the median duration of illness was 4 days (range 1–10 days). Symptoms included diarrhoea (100%), abdominal cramps (76%), vomiting (46%), and fever (46%). Seven of 10 student case-patients who submitted stool samples tested positive for Cryptosporidium; all five positive samples that amplified by PCR–RFLP were confirmed as C. parvum.
Interviews with students revealed that 66% of students reported wearing coveralls and 59% reported wearing boots; 97% reported being told to wash their hands after animal contact, but only 40% recalled being given specific instructions on how to wash their hands. In addition, 36% of students reported eating or drinking while on the farm campus.
In the univariate analysis, cleaning a calf pen, getting visible manure on one’s hands, having contact with any calf with scours, always wearing coveralls, wearing boots, hand washing with water only, and drying hands on one’s clothing were significantly associated with illness (Table 2).
Table 2.
Potential risk factors for cryptosporidiosis among students in the animal science class during the second outbreak, Minnesota, September 2003
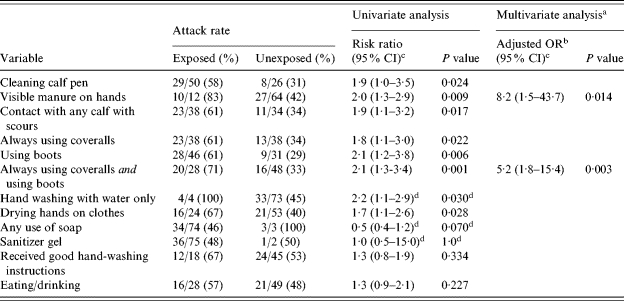
Variables included in the final multivariate analysis were: cleaning a calf pen; getting visible manure on one’s hands; having contact with any calf with scours; a combination variable of always using coveralls and using boots; hand washing with water only; any use of soap; and drying hands on clothing.
Because risk ratios were not calculable in the multivariate analyses, adjusted odds ratios were reported to provide an estimate for risk ratios in the multivariate analyses.
CI, Confidence interval.
Represents exact confidence interval and exact P value.
In the initial multivariate analysis, getting visible manure on one’s hands [adjusted odds ratio (aOR) 7·2, 95% confidence interval (CI) 1·4–38·2, P=0·020] and wearing boots (aOR 3·6, 95% CI 1·2–11·2, P=0·024) remained associated with illness. However, always wearing coveralls approached statistical significance (P=0·074), and there was confounding between always wearing coveralls, use of boots, and illness. Therefore, a combination variable of always wearing coveralls and using boots was created to replace the respective individual variables and control for confounding, and the multivariate analysis was repeated. In this analysis, getting visible manure on one’s hands and the combination variable of always wearing coveralls and using boots were the only variables that remained associated with illness (Table 2).
All of the variables that were associated with illness in the univariate analysis using case definition 1 were also associated with illness using case definition 2 (data not shown). In the multivariate analysis using case definition 2, getting visible manure on one’s hands (aOR 7·3, 95% CI 1·4–38·0, P=0·018) and the combination variable of always wearing coveralls and using boots (aOR 4·8, 95% CI 1·7–13·6, P=0·003) again were the only variables that remained associated with illness.
Ten students who were in the animal science class during the spring semester were enrolled in the animal science class again during the autumn semester. Of those 10 students, five (50%) had become ill during the spring semester, but none of the 10 became ill during the autumn semester. Other previous contact with farm animals (e.g. growing up on a farm) was not statistically protective against illness.
During this outbreak, illness attack rates differed significantly between the middle-school students (9th graders) and the high-school students (10, 11, and 12th graders): 27 out of 41 (66%) middle-school students were ill vs. 10 out of 36 (28%) high-school students (RR 2·4, 95% CI 1·3–4·2, P<0·001). In univariate analysis of middle-school students only, two risk factors were identified: drying hands on one’s clothing [13/15 (87%) who did this became ill vs. 14/26 (54%) who did not, RR 1·6, 95% CI 1·1–2·4, P=0·033], and eating/drinking while on the farm campus [13/15 (87%) who ate/drank became ill vs. 14/26 (54%) who did not, RR 1·6, 95% CI 1·1–2·4, P=0·033]. Among high-school students only, ‘receiving good hand-washing instructions’ was the only significant variable, and was protective against illness [1/15 (7%) students who reported receiving good instructions became ill vs. 9/20 (45%) who did not, RR 0·2, exact 95% CI 0·01–0·8, exact P=0·022].
In the subanalyses of different age groups using case definition 2, all of the variables that were significantly associated with illness using case definition 1 remained significant, and no additional significant variables were identified (data not shown).
The telephone interviews and environmental investigation provided insight into why substantial numbers of autumn semester students developed cryptosporidiosis after the implementation of our recommendations after the previous spring’s investigation. Students had been assigned to several sinks throughout the building, but hand washing was still cursory and poor. The coveralls and boots, jumbled in the closets, were washed every 1–2 weeks and were visibly soiled with manure. The coverall and boot closets were in the entry/exit hall, whereas the sinks were in the barn; students who wore the protective clothing changed out of it after washing their hands. Certain students brought their own protective clothing from home, but most of these students then returned the clothing to their backpacks after class. We also witnessed students eating food between classes from vending machines located in an adjacent building.
The farm manager had purchased a new group (n=15) of 3-day-old calves for the autumn semester; eight from a sales barn and seven from a private dairy farm. At the time of our environmental investigation, the calves were 3 weeks old; one had died of scours and several others appeared ill. Faecal specimens from 12 of the 14 remaining calves tested positive for at least one pathogen: Cryptosporidium (n=4), Cryptosporidium and Giardia (n=3), Cryptosporidium and Campylobacter coli (n=1), Giardia (n=2), C. coli (n=1), and Giardia and C. coli (n=1). None of the Cryptosporidium-positive samples amplified by PCR–RFLP.
DISCUSSION
This report documents two successive outbreaks of cryptosporidiosis at an educational farm programme for middle- and high-school students, resulting from direct contact with calves and lack of adequate hygiene practices by the students. Multiple potential risk behaviours were observed during the environmental investigation, including wearing street clothes and shoes, inadequate hand hygiene, removing soiled protective clothing after hand washing, and consumption of food and drink while on the farm campus. Failure to adequately and consistently implement our earlier recommendations probably contributed to the second outbreak. Our recommendations following the second investigation strongly emphasized the need for substantially improved student hygiene practices and for interaction with healthier, older calves. We recommended that:
calves be housed in physically separated pens and quarantined for 30 days prior to contact with students;
the first class of each semester be dedicated to educating students about the risk of enteric zoonotic pathogen transmission, and about proper hand-washing techniques and other appropriate hygienic practices;
detailed information be sent home to the students’ parents or guardians regarding zoonotic illness and specific instructions on how to properly handle/launder soiled clothing that students bring home;
hand washing be supervised and performed after removing the coveralls and boots;
the instructors end class periods early enough to allow students sufficient time to perform proper hand washing;
all eating/drinking in the building or on the bus on the way back to school be prohibited;
protective clothing and boots be laundered more frequently and stored properly to avoid cross-contamination.
These recommendations were implemented, and no enteric illnesses were identified in students of the class the following year.
Cryptosporidium is a well-established zoonotic pathogen, and is especially common among cattle. In 1993, the National Dairy Heifer Evaluation Program of the National Animal Health Monitoring System (NAHMS) (United States Department of Agriculture: APHIS:VS) conducted a survey of 1811 farms in 28 states, representing 78% of all US milking cows. Of 7369 calf faecal samples from 1103 farms, 22% of pre-weaned calves were shedding Cryptosporidium on any given day. The prevalence was highest (nearly 50%) among calves aged 1–3 weeks, and the parasite was estimated to be present on >90% of dairy farms [15]. A similar study of beef operations conducted in 1994 by NAHMS estimated that Cryptosporidium could be isolated from calves on ∼40% of beef operations [16]. Therefore, that calves were the source of the outbreaks described here is not surprising.
Although zoonotic infections through direct contact with farm animals have been reported with increasing frequency, the relative importance and burden of direct zoonotic cryptosporidiosis transmission is not entirely clear [6]. Contact with cattle is a known risk factor for cryptosporidiosis [17], and multiple outbreaks of cryptosporidiosis have been reported among veterinary students in academic veterinary settings [7–9]. Since 1990, a growing number of enteric disease outbreaks associated with animal contact settings (e.g. petting zoos, animal exhibits at county fairs, and educational farm programmes) have been reported from many countries [10, 11, 18, 19]. Along with E. coli O157, C. parvum has become one of the most commonly implicated pathogens in these outbreaks, with cattle, sheep, and goats the most commonly involved species. However, cryptosporidiosis outbreaks in the setting of public farm animal venues have been reported primarily in other countries, but not in the United States until recently [18].
Cryptosporidiosis is considerably under-recognized in the United States. A laboratory survey in 2000 revealed that 89% of laboratories did not include Cryptosporidium as part of the routine ova and parasite examination, and perform the test only if they have a special order to do so [20]. However, approximately three-fourths of clinicians assumed that the Cryptosporidium test was included in the ova and parasite examination [21]. Cryptosporidiosis also is under-detected because persons with diarrhoea often do not consult their health-care providers unless their illness is severe. During the spring outbreak reported here, only 29% of the case-patients had visited a health-care provider. During the autumn outbreak, no students sought care until a letter regarding illness among middle-school students was sent to parents. Lastly, providers usually do not order patient stool testing unless the illness is severe. Among the nine case-patients who visited a provider for their illness during the spring outbreak, only two had stool samples submitted for testing.
Transmission of Cryptosporidium and other enteric pathogens at public animal venues occurs through direct animal contact and from faecal contamination of food, water, or environmental surfaces. Several animal, environmental, and human factors increase the likelihood of transmission of enteric pathogens to persons at farm animal exhibits. The prevalence of some enteric pathogens is higher in immature animals, and most public farm animal venues prefer to exhibit young animals. In sales barns, from which many calves are obtained, calves are often deprived of colostrum and mingled with calves from multiple sources, increasing the likelihood of horizontal transmission of pathogens; these calves are probably more prone to illness than those obtained directly from private farms. Animals at exhibits may be more likely to shed enteric pathogens due to stress induced by transportation, confinement, dietary changes, and increased contact with people.
Visitors to public farm animal venues are often unaware of the potential risks associated with animal contact. Children <6 years of age, the most susceptible human population, are the most common participants at these types of venues and are the most likely to engage in hand-to-mouth activities (e.g. eating, thumb-sucking, and pacifier use) without appropriate hand washing first. Hand-washing facilities are often absent or inadequate. When unsupervised, children may wash their hands poorly.
In the United Kingdom, after the occurrence of multiple cryptosporidiosis and E. coli O157 outbreaks associated with farm visits in the early 1990s, several regional and national health authorities issued recommendations addressing the risks of zoonotic transmission of enteric pathogens at these venues [22–24]. In the United States, a CDC survey of state and territorial public health departments in 2000 determined that no state had established laws to control exposure of humans to enteric pathogens at public farm animal venues [19]. In 2001, CDC published national recommendations aimed at reducing the risk for transmission of enteric pathogens [25]. This was followed closely by publication of a compendium of measures to prevent disease and injury associated with animals in public settings, by the National Association of State Public Health Veterinarians (NASPHV); this set of guidelines was most recently updated in 2005 [26]. Both sets of guidelines recommend that venues minimize risk by separating animal and non-animal areas, facilitating hand washing, prohibiting all eating and drinking in animal-interaction areas, prohibiting the serving of unpasteurized milk, educating visitors about the risk of injury or enteric disease from animal contact, ensuring that high-risk populations observe heightened precautions, and using healthy animals with regular veterinary care.
Despite the availability of comprehensive recommendations, communication with venue operators about these guidelines, and their implementation, continue to be difficult. We previously investigated two enteric outbreaks in consecutive years from the same children’s farm day camp [18]. In both instances, calves were the source for the multiple enteric pathogens implicated in these outbreaks. Inadequate implementation of recommended prevention measures provided after the first outbreak at the day camp led to its recurrence. Thus, the cryptosporidiosis outbreaks described in this report represent the second instance in Minnesota where the failure to adequately and consistently implement recommended prevention measures contributed to recurrent illness.
As with many zoonotic disease problems, veterinarians are a critical public health resource regarding the safety of farm animal venues. Veterinarians serve as vital educational resources for venue operators and the attending public, and are in an influential and unique position to help petting zoos, educational farm programmes, and other animal exhibits remain safe for both animals and humans. Adhering to the existing guidelines would unquestionably minimize zoonotic pathogen transmission at these venues, if fully implemented. However, our experience demonstrates that simply communicating the guidelines to venue operators is insufficient. The public health community should strive to include practising veterinarians in their educational efforts about this issue, so that these veterinarians can work together with public health and venue operators to achieve the proactive implementation of existing recommendations.
ACKNOWLEDGEMENTS
Supported in part with funding provided through a cooperative agreement (U50/CCU511190) with the Centers for Disease Control and Prevention (CDC) as part of the Emerging Infections Program, Foodborne Diseases Active Surveillance Network (FoodNet). The authors thank Brian Lee and other members of the Minnesota Department of Health, Infectious Disease Epidemiology, Prevention, and Control Division who contributed to the outbreak investigations and who provided comments on the manuscript. We also thank Andrea Winquist and Anindya De of the CDC for their contributions to the statistical analysis and drafting of the manuscript.
DECLARATION OF INTEREST
None.
REFERENCES
- 1.Panciera RJ, Thomassen RW, Garner FM. Cryptosporidial infection in a calf. Veterinary Pathology. 1971;8:479–484. [Google Scholar]
- 2.Current WL et al. Human cryptosporidiosis in immunocompetent and immunodeficient persons. Studies of an outbreak and experimental transmission. New England Journal of Medicine. 1983;309:1252–1257. doi: 10.1056/NEJM198305263082102. [DOI] [PubMed] [Google Scholar]
- 3.Current WL. Human cryptosporidiosis. New England Journal of Medicine. 1983;309:1325–1327. doi: 10.1056/NEJM198311243092114. [DOI] [PubMed] [Google Scholar]
- 4.Guerrant RL. Cryptosporidiosis: an emerging, highly infectious threat. Emerging Infectious Diseases. 1997;3:51–57. doi: 10.3201/eid0301.970106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen XM et al. Cryptosporidiosis. New England Journal of Medicine. 2002;346:1723–1731. doi: 10.1056/NEJMra013170. [DOI] [PubMed] [Google Scholar]
- 6.Xiao L et al. Cryptosporidium taxonomy: recent advances and implications for public health. Clinical Microbiology Reviews. 2004;17:72–97. doi: 10.1128/CMR.17.1.72-97.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Levine JF et al. Cryptosporidiosis in veterinary students. Journal of the American Veterinary Medical Association. 1988;193:1413–1414. [PubMed] [Google Scholar]
- 8.Konkle DM, Nelson KM, Lunn DP. Nosocomial transmission of Cryptosporidium in a veterinary hospital. Journal of Veterinary Internal Medicine. 1997;11:340–343. doi: 10.1111/j.1939-1676.1997.tb00477.x. [DOI] [PubMed] [Google Scholar]
- 9.Pohjola S et al. Outbreak of cryptosporidiosis among veterinary students. Scandinavian Journal of Infectious Diseases. 1986;18:173–178. doi: 10.3109/00365548609032325. [DOI] [PubMed] [Google Scholar]
- 10.Bender JB, Shulman SA Reports of zoonotic disease outbreaks associated with animal exhibits and availability of recommendations for preventing zoonotic disease transmission from animals to people in such settings. Journal of the American Veterinary Medical Association. 2004;224:1105–1109. doi: 10.2460/javma.2004.224.1105. , and the Animals in Public Contact Subcommittee of the NASPHV. [DOI] [PubMed] [Google Scholar]
- 11.LeJeune JT, Davis MA. Outbreaks of zoonotic enteric disease associated with animal exhibits. Journal of the American Veterinary Medical Association. 2004;224:1440–1445. doi: 10.2460/javma.2004.224.1440. [DOI] [PubMed] [Google Scholar]
- 12.Isenberg HD. Clinical Microbiology Procedures Handbook. Washington, DC: American Society for Microbiology ASM Press; 1992. [Google Scholar]
- 13.Gibbons CL et al. Correlation between markers of strain variation in Cryptosporidium parvum: evidence of clonality. Parasitology International. 1998;47:139–147. [Google Scholar]
- 14.Olsvik O, Strockbine NA, Persing DH, Smith TF, Tenover FC, White TJ. Diagnostic Molecular Microbiology. Washington, DC: American Society for Microbiology; 1993. PCR detection of heat-stable, heat-labile, and Shiga-like toxin genes in Escherichia coli; pp. 271–276. : pp. [Google Scholar]
- 15.U.S. Department of Agriculture. Cryptosporidium is common in dairy calves. Fort Collins, CO: Centers for Epidemiology and Animal Health; 1993. Animal Plant Health Inspection Service: National Dairy Heifers Evaluation Project. [Google Scholar]
- 16.U.S. Department of Agriculture. Cryptosporidium and Giardia in beef calves. Fort Collins, CO: Centers for Epidemiology and Animal Health; 1994. Animal Plant Health Inspection Service: National Animal Health Monitoring System. [Google Scholar]
- 17.Roy SL et al. Risk factors for sporadic cryptosporidiosis among immunocompetent persons in the United States from 1999 to 2001. Journal of Clinical Microbiology. 2004;42:2944–2951. doi: 10.1128/JCM.42.7.2944-2951.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Smith KE et al. Outbreaks of enteric infections with multiple pathogens associated with calves at a farm day camp in consecutive years: public health implications. Pediatric Infectious Disease Journal. 2004;23:1098–1104. [PubMed] [Google Scholar]
- 19.Centers for Disease Control and Prevention. Outbreaks of Escherichia coli O157:H7 infections among children associated with farm visits – Pennsylvania and Washington, 2000. Morbidity and Mortality Weekly Report. 2001;50:293–297. [PubMed] [Google Scholar]
- 20.Jones JL et al. Survey of clinical laboratory practices for parasitic diseases. Clinical Infectious Diseases. 2004;38:S198–S202. doi: 10.1086/381587. [DOI] [PubMed] [Google Scholar]
- 21.Hennessy TW et al. Survey of physician diagnostic practice for patients with acute diarrhoea: clinical and public health implications. Clinical Infectious Diseases. 2004;38:S203–S211. doi: 10.1086/381588. [DOI] [PubMed] [Google Scholar]
- 22.Dawson A et al. Farm visits and zoonoses. Communicable Disease Report. CDR Review. 1995;5:R81–R86. [PubMed] [Google Scholar]
- 23.Health and Safety Executive. www.hse.gov.uk/pubns/ais23.pdf. www.hse.gov.uk/pubns/ais23.pdf Avoiding ill health at open farms – advice to farmers (with teachers’ supplement) ). Accessed 8 June 2004.
- 24.Middlesex – London Health Unit Investigation and Recommendations. www.healthunit.com/sitemaparchives.htm. www.healthunit.com/sitemaparchives.htm An E. coli O157:H7 outbreak associated with an animal exhibit, 1999. ). Accessed 8 June 2004.
- 25.Centers for Disease Control and Prevention. www.cdc.gov/ncidod/dbmd/outbreak/recomm_farm_animal.htm. www.cdc.gov/ncidod/dbmd/outbreak/recomm_farm_animal.htm Reducing the risk for transmission of enteric pathogens at petting zoos, open farms, animal exhibits, and other venues where the public has contact with farm animals. ). Accessed 8 May 2004.
- 26.Centers for Disease Control and Prevention. Compendium of measures to prevent disease associated with animals in public settings, 2005: National Association of State Public Health Veterinarians, Inc. (NASPHV) Morbidity and Mortality Weekly Report. 2005;54:1–13. [PubMed] [Google Scholar]