SUMMARY
Vibrio parahaemolyticus and Vibrio vulnificus were isolated from faecal samples of wild aquatic birds in winter. Although V. parahaemolyticus and V. vulnificus were present in low numbers in seawater in the area where the faecal samples of the birds were collected, the pathogens were isolated from the faeces of the birds. This study demonstrates that wild aquatic birds are a vehicle for V. parahaemolyticus and V. vulnificus to survive in winter.
Vibrio parahaemolyticus and Vibrio vulnificus are important pathogens of the Vibrio genus. It is known that Vibrio infections occur through the consumption of contaminated seafoods and contact of wounds with contaminated seawater. In Japan, V. parahaemolyticus is one of the most common pathogens of foodborne infections. More than 94 cases of V. vulnificus infection including 68 deaths were reported during 1998–2003, and an average occurrence of 425 cases per year has been estimated [1]. Contaminations of seafood and seawater with V. vulnificus have also been reported in Japan [2–4]. To avoid infection, the investigation of the distribution of pathogens in the environment is important.
Population size of V. parahaemolyticus and V. vulnificus in seawater is correlated with seawater temperature [5–7], and most infections by V. parahaemolyticus or V. vulnificus occur during summer. It was believed that these pathogens were dead during cold weather conditions. However, recent studies have indicated that cold temperatures induce a ‘viable but non-culturable (VNC)’ state in these pathogens [8, 9]. Moreover, the possibility that V. vulnificus overwinters in certain conditions has also been indicated [10]. DePaola et al. [11] isolated pathogens from fish at higher densities than in sediment or seawater during winter. Previously, a few reports stated that vibrios, including V. cholerae and V. parahaemolyticus, were isolated from aquatic birds such as the gull [12–14]. However, the isolation of V. vulnificus from the faeces of birds has never been reported. Furthermore, there are a few reports on the isolation of V. vulnificus from seawater or enrichment samples in the winter season. This study demonstrates that wild aquatic birds are a vehicle of V. vulnificus and V. parahaemolyticus in winter.
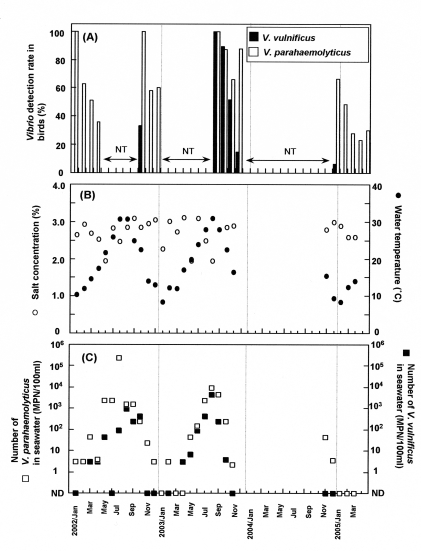
A total of 616 fresh faecal samples from birds such as the black-tailed gull (Larus carassirostris), herring gull (Larus argentatus), black-headed gull (Larus ridibundus), mallard (Anas platyrhynchos), European widgeon (Anas penelope), and common teal (Anas crecca), were collected along a section of coastline in Kumamoto prefecture, southern Japan, from January to April 2002, October to December 2002, August to November 2003, in December 2004, and January to April 2005 (Table 1, Fig.). Because herring gulls/black-tailed gulls and black-headed gulls did not visit the area between May and July, we could not collect their faecal samples during this period. Immediately after each group of herring gulls/black-tailed gulls or black-headed gulls left from the breakwater, their faecal samples were quickly collected, and ∼1 g of each was put into 10 ml alkaline peptone water (APW). The samples were transported immediately to our laboratory without cooling within 1 h. To detect V. parahaemolyticus and V. vulnificus, each sample in 10 ml of APW was incubated at 35°C for 18 h. One loop (10 μl) of the culture was inoculated onto CHROMagar Vibrio (CHROMagar, Paris, France) and incubated at 35°C for 18 h. Colonies suspected as V. parahaemolyticus and V. vulnificus were confirmed by the oxidase test, culture in triple sugar iron agar medium (Nissui Pharmaceutical Co. Ltd, Tokyo, Japan) containing 2% NaCl, culture in lysine indol motility medium (Nissui Pharmaceutical Co. Ltd) containing 2% NaCl, and culture in VP semi-solid medium (Nissui Pharmaceutical Co. Ltd) containing 2% NaCl, and growth in Nutrient broth (Oxoid, Hampshire, UK) containing 0, 3, 8 and 10% NaCl. Furthermore, the suspected colonies were tested for the presence of the cytotoxin-haemolysin gene of V. vulnificus, and the tdh and trh genes of V. parahaemolyticus by the PCR method with the primer set used by Hill et al. [15] and Tada et al. [16] respectively. As a result, V. parahaemolyticus was detected in bird faecal samples at high levels on most sampling dates even in winter [Fig. (a)]. V. vulnificus was detected in October 2002, between August and November 2003, and in December 2004.
Table 1.
Detection of V. vulnificus and V. parahaemolyticus in wild aquatic birds in Japan

VP, Vibrio parahaemolyticus; VV, Vibrio vulnificus.
Number of detected sample/total number of tested sample.
Mallard.
Common teal.
Ten European widgeon and nine common teal.
A TRH-positive strain (O10:KUT) was isolated.
Fig.
Detection of V. parahaemolyticus and V. vulnificus in bird faecal samples and the environment. (a) The detection ratio of Vibrio in the bird faecal samples; NT, not tested. (b) The salt concentration and water temperature of seawater near the place the bird faecal samples were collected. (c) The numbers of V. parahaemolyticus and V. vulnificus in seawater; ND, not detected.
The seawater samples were collected in sterilized plastic bottles in areas where the faecal samples of the birds were also collected. Salinity was measured with a salt analyser (SAT-210, Toa Electronics, Tokyo, Japan). The temperature of seawater was measured with a thermometer. Seawater salinity (19–31‰) was not constant throughout the four seasons [Fig. (b)]. Seawater salinity might be affected by weather or other factors rather than seasonal factors, while the temperature seemed to be affected by seasonal factors. The temperature was <15°C between December and March and >24°C between June and September [Fig. (b)]. Most bird faecal samples were collected in periods when the seawater was <15°C [Fig. (a, b)].
The numbers of V. parahaemolyticus and V. vulnificus in the seawater samples were estimated by the three-tube most probable number (MPN) method. Volumes of 10 ml and 1 ml of seawater were added to 10 ml of double-strength APW, and APW respectively. One ml of dilutions (10−1–10−4) in PBS was added to 10 ml APW. In addition, 500 ml of each seawater sample was filtered with a filter (pore size: 0·45 μm) and 40 ml APW was added to a tube contained with the filter. After incubation at 35°C for 18 h, 10 μl of the culture was streaked onto CHROMagar Vibrio and incubated at 35°C for 18 h. The suspected colonies were confirmed by the method described above. The results showed that the population number of V. parahaemolyticus in seawater samples was associated with temperature [Fig. (c)]. The population was <100 MPN/100 ml between November and April and >1000 MPN/100 ml between July and September. The population number of V. vulnificus was also associated with seasonal factors and tended to be lower than that of V. parahaemolyticus. The population number of V. vulnificus was <10 MPN/100 ml and >100 MPN/100 ml between November–April and July–September respectively [Fig. (c)]. The number of vibrios in water seems to be related to temperature. Vibrios were isolated from bird faecal samples even if they were in low numbers in seawater. V. parahaemolyticus was detected with a higher rate than V. vulnificus [Fig. (a, b)] both in bird faecal samples and seawater.
The details of detection of vibrios in birds are shown in Table 1. V. parahaemolyticus was detected at the highest rate in herring gull/black-tailed gull samples (more than 67·5% of samples) (Table 1). The rates of V. parahaemolyticus detection in black-headed gulls and ducks were 54·4 and 33·3% respectively. V. vulnificus was detected at the highest rate in herring gulls/black-tailed gulls (>26·9%). However, the rates of V. vulnificus detection in black-headed gulls and ducks were 0·8 and 0% respectively. In all bird faecal samples, the detection rate of V. parahaemolyticus (55·4%) was higher than that of V. vulnificus (14·1%).
Serotyping of V. parahaemolyticus was performed for 117 strains of V. parahaemolyticus isolated between January and April 2002 by the agglutination test with antisera against O and K antigens (Denka Seiken Co. Ltd, Tokyo, Japan). As a result, various combinations of O and K serotypes were observed (Table 2). More than half of the strains had untypable K antigenicity. It is suggested that many unknown types of antigenicity exist in the environment. Although tdh-positive strain was not isolated through this study, a trh-positive strain (serotype O10:KUT) was isolated from ducks in February 2005. Demonstrating that aquatic birds can carry pathogenic strains of V. parahaemolyticus.
Table 2.
Serotypes of Vibrio parahaemolyticus isolates in January–April, 2002
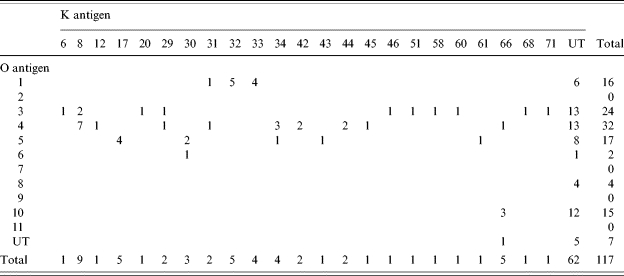
UT, Untypable.
Aquatic birds catch and eat shellfish at low tide. At high tide, herring gulls/black-tailed gulls and black-headed gulls stay around the breakwater in separate groups. Aquatic birds spit out their undigested food on the breakwater. This undigested food on the breakwater makes it easy to identify what they feed on. Their food includes small crab, small squilla and other shellfish. These shellfish may carry V. vulnificus and V. parahaemolyticus, and infect birds through ingestion. V. parahaemolyticus was also detected in the faecal samples of birds that consumed seaweed (i.e. mallard, European widgeon and common teal). This suggests that the number of V. parahaemolyticus in seaweed was similar to that in shellfish.
The number of cases of V. vulnificus infection in Kumamoto prefecture were one, three and two during the years 2002, 2003 and 2004 respectively. Furthermore >100 MPN/100 ml of V. vulnificus was detected in seawater in the summer, between July and September, of every year. These facts indicate the existence of vehicles for the pathogen to survive in water. In this study, we analysed aquatic bird faecal samples for the presence of V. parahaemolyticus and V. vulnificus in order to understand their role in the presence of these pathogens in seawater or shellfish in winter. The detection rate in the faecal samples showed a decline with the decline in the detection rate in seawater. However, V. parahaemolyticus and V. vulnificus were sometimes isolated only from the faecal samples of birds and not from nearby seawater. Birds might act as a potential vehicle in the transmission of these pathogens. It is not known whether vibrios colonize the birds’ intestines. However, V. parahaemolyticus and V. vulnificus were easily recovered from part of the samples in this study by direct plating of bird faeces (data not shown). This indicates the possibility of the growth of vibrios in the intestine. Further study should concentrate on the growth of vibrios in bird intestine. In addition, during winter vibrio cells existing in a VNC state might change to a culturable state once they enter the intestine of aquatic birds. It seems that environments such as seawater or shellfish might be exposed with the culturable cells.
ACKNOWLEDGEMENTS
This work was supported by a Health Sciences Research Grant from the Ministry of Health, Labor and Welfare, Japan.
DECLARATION OF INTEREST
None.
REFERENCES
- 1.Osaka K et al. Vibrio vulnificus septicaemia in Japan: an estimated number of infections and physicians’ knowledge of the syndrome. Epidemiology and Infection. 2004;132:993–996. doi: 10.1017/s0950268804002407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Oonaga K et al. Basic studies on Vibrio vulnificus infection: isolation of V. vulnificus from sea water, sea mud, and oysters [in Japanese] Kansenshogaku Zasshi. 2002;76:528–535. doi: 10.11150/kansenshogakuzasshi1970.76.528. [DOI] [PubMed] [Google Scholar]
- 3.Oonaga K et al. A basic study of Vibrio vulnificus infection: serotyping and drug sensitivity test of environment-derived strains and human clinical isolates [in Japanese] Kansenshogaku Zasshi. 2004;78:83–89. doi: 10.11150/kansenshogakuzasshi1970.78.83. [DOI] [PubMed] [Google Scholar]
- 4.Takahashi H et al. Development of a quantitative real-time polymerase chain reaction targeted to the toxR for detection of Vibrio vulnificus. Journal of Microbiological Methods. 2005;61:77–85. doi: 10.1016/j.mimet.2004.11.005. [DOI] [PubMed] [Google Scholar]
- 5.Kelly MT. Effect of temperature and salinity on Vibrio (Beneckea) vulnificus occurrence in a Gulf coast environment. Applied and Environmental Microbiology. 1982;44:820–824. doi: 10.1128/aem.44.4.820-824.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tilton RC, Ryan RW. Clinical and ecological characteristics of Vibrio vulnificus in the Northeastern United States. Diagnostic Microbiology and Infectious Disease. 1987;6:109–117. doi: 10.1016/0732-8893(87)90094-0. [DOI] [PubMed] [Google Scholar]
- 7.Kaneko T, Colwell RR. Distribution of Vibrio parahaemolyticus and related organisms in the Atlantic ocean off South Carolina and Georgia. Applied Microbiology. 1974;28:1009–1017. doi: 10.1128/am.28.6.1009-1017.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jiang XP, Chai TJ. Survival of at low temperatures under starvation conditions and subsequent resuscitation of viable, nonculturable cells. Applied and Environmental Microbiology. 1996;62:1300–1305. doi: 10.1128/aem.62.4.1300-1305.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wolf PW, Oliver JD. Temperature effects on the viable but nonculturable state of Vibrio vulnificus. FEMS Microbiology Letters. 1992;101:33–39. [Google Scholar]
- 10.Oliver JD et al. Entry into, and resuscitation from, the viable but nonculturable state by Vibrio vulnificus in an estuarine environment. Applied and Environmental Microbiology. 1995;61:2624–2630. doi: 10.1128/aem.61.7.2624-2630.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.DePaola A, Capers GM, Alexander D. Densities of Vibrio vulnificus in the intestines of fish from the U.S. Gulf Coast. Applied and Environmental Microbiology. 1994;60:984–988. doi: 10.1128/aem.60.3.984-988.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Buck JD. Isolation of Canada albicans and halophilic Vibrio spp. from aquatic birds in Connecticut and Florida. Applied and Environmental Microbiology. 1990;56:826–828. doi: 10.1128/aem.56.3.826-828.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lee JV et al. The incidence of Vibrio cholerae in water, animal and birds in Kent, England. Journal of Applied Bacteriology. 1982;52:281–291. doi: 10.1111/j.1365-2672.1982.tb04852.x. [DOI] [PubMed] [Google Scholar]
- 14.Schlater LK et al. A non-O1 Vibrio cholerae from a goose. Avian Disease. 1980;25:199–201. [PubMed] [Google Scholar]
- 15.Hill WE et al. Polymerase chain reaction identification of Vibrio vulnificus in artificially contaminated oysters. Applied and Environmental Microbiology. 1991;57:707–711. doi: 10.1128/aem.57.3.707-711.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tada JT et al. Detection of the thermostable direct hemolysin gene (tdh) and the thermostable direct hemolysin-related hemolysin gene (trh) of Vibrio parahaemolyticus by polymerase chain reaction. Molecular and Cellular Probes. 1992;6:477–487. doi: 10.1016/0890-8508(92)90044-x. [DOI] [PubMed] [Google Scholar]