SUMMARY
Vaccination coverage in 595 adult patients undergoing total splenectomy in the Hospital Clinic of Barcelona during 1992–2002 was studied. The rates of cover for pneumococcal, Haemophilus influenzae type b and meningococcal vaccines were 63, 63 and 61% respectively, during 2000–2002; 32, 17 and 22% in 1997–1999 and 24, 9 and 8% in 1992–1996. Multivariate analysis showed a greater risk of no vaccination in splenectomies due to trauma, malignant neoplasms of solid organs and incidental splenectomy compared with both neoplastic and non-neoplastic haematological disease, and those patients undergoing splenectomy before 2001. Coverage ( 1 vaccine) since 1997 in patients with haematological diseases was 83·5% (71/85), haematological neoplasias 69·2% (18/26), solid organ neoplasms 38·3% (36/94), incidental splenectomy 35·6% (16/45), and traumas 28·4% (21/74). Mandatory hospital admission of patients undergoing splenectomy offers a good opportunity for vaccination of these patients. Specific vaccination policies should be developed to take advantage of this circumstance.
1 vaccine) since 1997 in patients with haematological diseases was 83·5% (71/85), haematological neoplasias 69·2% (18/26), solid organ neoplasms 38·3% (36/94), incidental splenectomy 35·6% (16/45), and traumas 28·4% (21/74). Mandatory hospital admission of patients undergoing splenectomy offers a good opportunity for vaccination of these patients. Specific vaccination policies should be developed to take advantage of this circumstance.
INTRODUCTION
Overwhelming post-splenectomy infection (OPSI) is the most severe long-term complication of splenectomy and is defined as fulminant sepsis or meningitis with septic shock and disseminated intravascular coagulation. It has a case-fatality rate of ∼50% [1] and an estimated incidence of 0·04–0·18/100 persons per year [2, 3] with the risk being greater in the first 2 years post-splenectomy, although it persists throughout life. Severe post-splenectomy infection (defined as meningitis, septicaemia or pneumonia requiring hospitalization) has a still greater incidence of 0·42–7·16/100 persons per year [2, 3].
The main agents involved are capsulated microorganisms, including Streptococcus pneumoniae (pneumococcus) (57%), Haemophilus influenzae type b (6%), and Neisseria meningitidis (meningococcus) (4%) [4]. The spectrum changes according to age, with more pneumococcal infections in older people. However, Gram-negative infections such as Escherichia coli and Pseudomonas aeruginosa are increasingly frequent and also have a high case-fatality rate [5].
Splenectomy is the main cause of hyposplenism [6]. Splenectomies are carried out for three main reasons: rupture of the spleen (trauma, intraoperative, or spontaneous rupture), as a treatment for various blood disorders (haemolytic syndromes, co-adjuvant to some haematological neoplasms, etc.), and resections of splenic tumours or neoplasms of adjacent organs [7]. The estimated prevalence of splenectomized individuals in the general population is 9·75/10 000 [8], a relatively large group of patients in a potentially severe situation.
To reduce the incidence of fulminant infections in this risk group, guides and recommendations for systematic preventive measures have been designed, based on vaccination, antibiotic prophylaxis and health education [9–11]. However, at present, there are no national recommendations for the management of post-splenectomy infection in Spain. The vaccines currently recommended in splenectomized adults in our setting are the 23-valent pneumococcal polysaccharide (PN23), the conjugated meningococcal C (MCC) and the Haemophilus influenzae type b (Hib) vaccines. If the surgery is elective, vaccination should be carried out at least 2 weeks before the intervention to achieve an optimal humoral response. If this is not possible, in order not to lose any opportunity for vaccination, the patient should be vaccinated as soon as practically possible after the operation and, in all cases, before hospital discharge.
From the 1990s, various reports have indicated defects in compliance with these recommendations. Reported vaccination coverage rates differ considerably according to which country, the period studied, the type of vaccine (the most studied is the pneumoccocal vaccine) and the underlying disease. The reported coverage of the pneumococcal vaccine varies between 60 and 91% [12, 13].
The objective of this paper was (i) to describe the evolution of vaccination coverage rates of the pneumococcal, meningococcal and Hib vaccines in patients undergoing total splenectomy in the Hospital Clinic of Barcelona between 1992 and 2002, (ii) to evaluate compliance with recommendations in relation to the time intervals between vaccination and the operation, and (iii) to identify the main determining factors of vaccination coverage.
METHODS
We carried out a cross-sectional study in patients undergoing total splenectomy in the Hospital Clinic of Barcelona between 1992 and 2002. The Hospital Clinic is a reference hospital for an adult population of 370 000, with 770 beds, ∼35 700 admissions a year with an average stay of ∼7 days, and 12 500 annual operations with general anaesthesia. Since 1997, the hospital has applied a protocol consisting of concomitant vaccination with the PN23, meningococcal A-C (MAC) (until 2000), MCC (from 2000) and Hib, in addition to reviewing the other routine adult vaccinations. Moreover, from 2000, a network for the vaccination of these patients was established in coordination with the main services involved (Preventive Medicine, Haematology, Trauma and General Surgery).
The Medical Records Department provided details of all patients undergoing procedure 41·5 of the ICD-9-CM (total splenectomy) during the study period. Patients aged <18 years at the time of the operation were excluded. Demographic variables (date of birth and sex), clinical variables (main diagnosis at discharge classified by ICD-9-CM and death) and administrative variables (dates of admission, discharge and operation, discharge service, types of admission and discharge) were collected for each patient.
The vaccination status of patients was determined using the database of the Adult Vaccination Centre of the Hospital Clinic, which contains details of vaccines administered and the date of administration for all patients seen since 1992. Information relative to the PN23, MAC, MCC and Hib vaccines was obtained for each patient studied and linked with medical record data by means of the hospital’s medical history identification code.
The reasons for splenectomy were classified in five main categories based on underlying illnesses (ICD-9-CM codes in parentheses). We classified malignant neoplasms of adjacent solid organs: including malignant neoplasms of digestive organs and peritoneum (150–159), ovary (183), kidney (189), adrenal gland (194·0) and digestive system metastasis (197·6, 197·8), in group 1. Group 2 comprised malignant haematological neoplasms with splenic involvement: Hodgkin’s disease, non-Hodgkin’s lymphomas and leukaemia (201–205) and some haematological neoplasms of uncertain behaviour (238·7). Group 3 included non-neoplastic haematological disease: haemolytic and aplastic anaemias (282–284), thrombocytopathies (287), spleen disorders (289·4–289·5, 759·0, 789·2) and infectious or rheumatological diseases with splenic involvement (017·7, 023, 038, 042, 277, 279). Group 4, incidental splenectomies for surgical procedures, included benign neoplasms of the digestive system (211), gastrointestinal haemorrhage and vascular disorders (578, 241–242, 452–453), gastric and duodenum ulcers (531–532), intestinal disorders (556–558, 560–562, 569), biliary and liver disorders (571–576), diseases of the pancreas (577). Group 5 comprised trauma and external injuries (801–868), primarily injury to spleen (865).
Statistical analysis was performed using the SPSS version 11 statistical package (SPSS Inc., Chicago, IL, USA). Vaccination status was determined using the binary variable of no vaccination (1=unvaccinated, 0=vaccinated, defined as having received at least one of the indicated vaccines). The association of the binary variable with age, sex, year of splenectomy, aetiological group, days of hospital stay and death was studied. In the bivariate analysis, statistical significance was established at P<0·05. The χ2 test was used for categorical variables and Fisher’s exact test was used when the χ2 test could not be applied. The linear trend test was used for ordinal variables. For the multivariate analysis, a logistic regression model was used that included the independent variables which were statistically significant in the bivariate analysis.
RESULTS
In total, 595 splenectomies were carried out between 1992 and 2002, an average of 54 per year (range 43–66); 60·3% were male (n=359). The average age was 51·5 years (s.d.=20), with a bimodal distribution consisting of a peak between 20–30 years of age and another between 50–75 years. The peaks were greater in males but also occurred in female patients.
The most frequent disorders involved were malignant neoplasms of solid organs (173), non-neoplastic haematological diseases (163), trauma (134), incidental splenectomies (75) and malignant haematological neoplasms (50). Table 1 shows the demographic characteristics (age and sex) and the variables related to the hospital admission in which the splenectomy was carried out (deaths in the same admission and the days of hospital stay). The number of total deaths refers to deaths during the hospital admission in which the splenectomy was carried out and deaths in subsequent admissions to the Hospital Clinic of Barcelona. Death was recorded in the Hospital Clinic in 110 out of 595 (18·5%) of the cases. In 57 (51·8%) of these, death occurred during the hospitalization in which the total splenectomy was carried out, and in 53 (49·2%) in posterior admissions. Deaths occurring in other health centres or in the community were not included.
Table 1.
Main characteristics of patients undergoing total splenectomy in the Hospital Clinic of Barcelona, 1992–2002
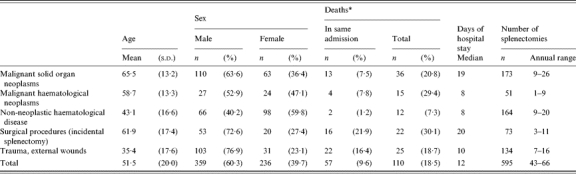
s.d., Standard deviation.
The overall coverage for the period studied (minimum of one indicated vaccine) was 38·3%. There were important differences in annual coverage, with a sustained increase from 1992 (7·0% vaccinated) to 2002 (83·3%) (Table 2). The table also shows patients vaccinated in the same year as the splenectomy and those vaccinated in posterior years, and clearly shows that a considerable part of the coverage achieved during the first years of the study was due to vaccination in posterior years.
Table 2.
Vaccine coverage rates with respect to the year of splenectomy
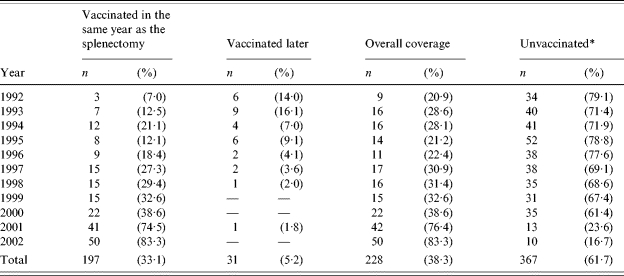
Unvaccinated according to the register of the Adult Vaccination Centre of the Hospital Clinic.
Table 3 shows the differences in vaccination coverage between the different aetiological groups, and is divided into three periods, 1992–1996 (no protocol), 1997–1999 (vaccination protocol) and 2000–2002 (vaccination network). Analysis of coverage rates obtained from 1997 to 2002 revealed that the coverage was 83·5% (71/85) in patients with haematological diseases, 69·2% (18/26) for haematological neoplasms, 38·3% (36/94) for solid organ neoplasias, 35·6% (16/45) for incidental splenectomy, and 28·4% (21/74) for traumas.
Table 3.
Vaccination coverage rates according to aetiological group and period of splenectomy
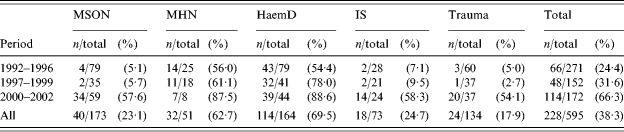
MSON, malignant solid organ neoplasms; MHN, malignant haematological neoplasms; HaemD, Non-neoplastic haematological disease; IS, incidental splenectomy.
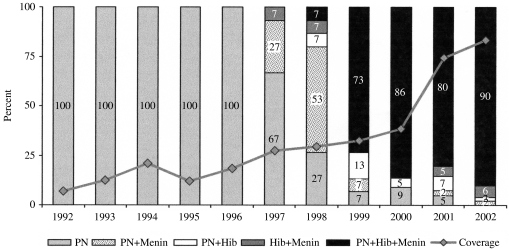
The vaccines administered differ according to the year of administration, the protocols established and the availability of vaccines. Figure 1 shows the number of vaccinations at the first visit as a percentage of the total of vaccinated patients. From 1992 to 1996, the only vaccine administered was PN23, whereas in 2002, the three indicated vaccines were administered concomitantly to 90% of patients. In 1997, the vaccination protocol was introduced, with the inclusion of the meningococcal vaccine (MAC and, from 2000, MCC), and Hib.
Fig. 1.
Vaccines administered in the first visit to the vaccination centre according to the year of administration. Percentage of vaccines administered in the same year. PN, pneumococcal. Hib, H. influenzae type b. Menin, meningococcal vaccine (MAC or MCC). The coverage indicates the percentage of patients vaccinated in the same year as the splenectomy.
Twenty-one per cent (12/58) of patients vaccinated only with PN23 also received the Hib and meningococcal vaccines in subsequent visits. Among patients receiving the MAC vaccine 13·6% (9/66) also received the MCC vaccine.
One third (24/72) of patients in whom a second dose of the PN23 vaccine at 5 years was recommended, made an appointment to receive the second dose. The average time between administration of the first and second doses of the PN23 vaccine was 5·07 years (range 4·03–6·51 years).
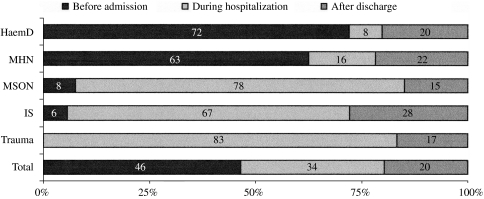
Figure 2 shows important variations in the moment of vaccination according to the underlying disease. Patients with haematological diseases received vaccination earlier than others, since the majority are sent to the vaccination centre before the intervention. Vaccination before surgery occurred in 63% of the cases of haematological neoplasms and in 72% of non-neoplastic haematological diseases. Vaccination was carried out during the hospital admission in which the splenectomy was carried in the majority of cases involving trauma (78%), incidental splenectomy (67%) and neoplasms of solid organs (83%).
Fig. 2.
Percentage distribution of vaccinations with respect to vaccination before, during or after admission for splenectomy for each aetiological group. HaemD, Non-neoplastic haematological disease; MHN, malignant haematological neoplasms; MSON, malignant solid organ neoplasms; IS, incidental splenectomy.
In patients vaccinated before surgery (n=106), the average number of days before surgery was 23. In patients vaccinated during the same admission, the average time was 2 days after surgery (n=77) and in patients vaccinated after hospital discharge the average time of vaccination was 4·5 years after discharge (including patients splenectomized between 1992 and 1997 who received posterior rescue doses).
In both the bivariate and multivariate analyses, the associations were calculated globally and for each one of the three periods in which vaccination procedures were modified (1992–1996, 1997–1999 and 2000–2002). In the multivariate analysis the following statistically significant variables in the bivariate analysis were included: age, sex, days of hospital stay, death during the admission, aetiological group and year of the splenectomy. The only statistical association that was maintained for the three periods studied was the greater risk of no vaccination in splenectomies due to trauma, malignant neoplasms of solid organs and incidental splenectomy compared with both neoplastic and non-neoplastic haematological disease (ORs 13·5, 12·7 and 26·3 respectively, P<0·01 in all cases). During the period 2000–2002, there was a greater risk of not being vaccinated in patients who died during the hospital admission (OR 20·1, P=0·002). The differences observed in the coverage of years 2002 and 2001 with respect to the years before 2000 were statistically significant both in the global multivariate analysis (OR 55·3, P<0·001) and in the linear trend test in the bivariate analysis.
DISCUSSION
Reports on the prevention of infection in splenectomized patients in Spain are limited to a single study [14]. Recent fatal cases of fulminant sepsis in splenectomized young adult patients in our centre show that the problem, although infrequent, can have a great impact on patients and their families. The existence of preventive recommendations means that these infections should be considered as avoidable. The continuous appearance of cases suggests possible deficiencies in the application of these measures and indicates the need to study their possible causes in order to make interventions more effective. Thus, given the limited data available, determining the current state of care of these patients and collecting the minimum data necessary to hypothesize on possible improvements in care is necessary.
Studies of vaccination coverage in splenectomized patients shows considerable disparities according to country, type of vaccine studied, type of surgery, and underlying diseases [12, 13, 15–24]. Coverage ranged from 14 to 91% for the pneumococcal vaccine, but rose to 60–91% when only studies carried out in the 1990s were considered [13, 15–17, 21–24]. Differences in the methodology and the populations studied mean these coverage rates are not totally comparable in epidemiological terms, although they provide valuable indicative information.
In Spain, Galán et al. [14] found coverage rates of 62·7% (PN23), 32·8% (Hib) and 7·5% (MAC) in 73 patients in Minorca. They found better coverage in haematological patients (94% for PN23) with respect to those splenectomized due to incidental causes (50%) and trauma (56%). Kyaw et al. [15], in Scotland, found coverage rates of 88% (PN23), 70% (Hib) and 51% (MAC) in 708 patients studied between 1988 and 1998 whereas in England MacInnes et al. [13] reported coverage rates of 91, 79, and 80% for pneumococcus, H. influenzae and meningococcus respectively, in 258 patients between 1992 and 1994. This difference may be due to the fact that different areas of the United Kingdom have promoted specific programmes for the detection and implementation of preventive measures in asplenic patients, such as the creation of registers [6] and strategies (update and promotion of management guides in health workers, distribution of alert cards, the use of checklists) [13].
In this light, the coverage rates achieved in our centre in 2002 (83·3%) can be considered satisfactory although not optimal. This level of coverage has only been achieved since 2001 (76·4%), with much lower rates in previous years (range 20·9–38·6%). This is confirmed by the multivariate analysis, which showed that undergoing a splenectomy before 2001 entailed a greater risk of no vaccination. The coverage rates observed during the 1990s comprised mainly haematological patients (both neoplastic and non-neoplastic). This suggests that the haematology department has been successful in the management of asplenia and, in contrast, better preventive measures in other departments managing asplenic patients are needed. However, the contact of surgical departments with this type of patient is basically limited to the operation itself. For this reason, a strategy whereby the physician responsible for the patient (oncologists, general practitioners, etc.) is responsible for carrying out the recommended vaccination strategies seems advisable. With respect to the time between vaccination and surgery, it seems that the currently recommended times are complied with, i.e. an average of 23 days before surgery and 2 days after surgery, as shown in the results.
One of the limitations of the study and a possible source of bias is that the data were obtained from only one source, a hospital vaccination centre, thereby possibly disregarding other possible sources of vaccination such as primary health care. Patients may be counted as unvaccinated when in fact they have received vaccination from another source. However, the probability of bias is low, since, in Catalonia, primary health-care centres did not routinely administer the PN23 vaccine until the year 2000, with the vaccine being administered almost exclusively in hospitals. This, together with the fact that more than one vaccine is recommended (Hib and MAC or MCC, which are not as widely administered as PN23), means that all those patients who might have been vaccinated in other centres, would, in fact have been sent to our Adult Vaccination Centre in accordance with current recommendations on vaccine administration.
Another limitation could be the lack of data on deaths outside the hospital. A large percentage of splenectomized patients have an underlying malignant neoplasm and may, therefore, be supposed to have a lower survival rate than other splenectomized patients. This may reduce the possibility of these patients attending the vaccination centre later, if they have not been vaccinated before or during the admission. Death during the hospital stay was associated with lower rates of vaccination in the statistical analysis. This may be because death occurs a short time after surgery, before vaccination, or because vaccination is postponed due to the critical state of the patient. Further study is needed to clarify this point.
As splenectomy requires hospitalization, a policy of vaccinating patients during their hospital stay is the key to fulfilling current recommendations on preventive vaccinations. However, primary health-care centres are also involved in these recommendations, and better coordination through discharge reports which clearly state the procedures and treatments administered would better educate the patient about their new health status [16, 17, 25]. In Spain, there is increasing pressure to raise vaccination coverage rates and improve the management of these patients. Gudiol [26], in an editorial, suggests the creation of a register which would enable mortality and morbidity studies of asplenic patients and their long-term follow-up.
The main consequence of low vaccination coverage is a pool of splenectomized patients without vaccination and, therefore, with a greater risk of severe infection. A means should thus be found to offer this group of patients the current recommended preventive measures available, including vaccination. In addition, improved health education is necessary to ensure these patients are aware of the risks they are exposed to and the need to convey their status as asplenic patients to all health professionals with whom they are in contact. It should also be remembered that current polysaccharide vaccines have a limited efficacy in immunosuppressed patients (haematological neoplasms). This suggests the need for new and more effective conjugated pneumococcal vaccines [27].
The results of this study may help to improve the strategies established in the hospital in order to achieve a better compliance with current recommendations. However, there is a need to design and evaluate specific interventions that will homogenize criteria and actions for all asplenic patients.
DECLARATION OF INTEREST
None.
REFERENCES
- 1.Bisharat N et al. Risk of infection and death among post-splenectomy patients. Journal of Infection. 2001;43:182–186. doi: 10.1053/jinf.2001.0904. [DOI] [PubMed] [Google Scholar]
- 2.Cullingford GL et al. Severe late postsplenectomy infection. British Journal of Surgery. 1991;78:716–721. doi: 10.1002/bjs.1800780626. [DOI] [PubMed] [Google Scholar]
- 3.Schwartz PE et al. Postsplenectomy sepsis and mortality in adults. Journal of the American Medical Association. 1982;248:2279–2283. [PubMed] [Google Scholar]
- 4.Holdsworth RJ, Irving AD, Cuschieri A. Postsplenectomy sepsis and its mortality rate: actual versus perceived risks. British Journal of Surgery. 1991;78:1031–1038. doi: 10.1002/bjs.1800780904. [DOI] [PubMed] [Google Scholar]
- 5.Ejstrud P et al. Risk and patterns of bacteraemia after splenectomy: a population-based study. Scandinavian Journal of Infectious Diseases. 2000;32:521–525. doi: 10.1080/003655400458811. [DOI] [PubMed] [Google Scholar]
- 6.Spickett GP et al. Northern region asplenia register – analysis of first two years. Journal of Clinical Pathology. 1999;52:424–429. doi: 10.1136/jcp.52.6.424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Polo Sabau J et al. Splenectomy indications at a general hospital. Revista Clinica Espanola. 1999;199:126–131. [PubMed] [Google Scholar]
- 8.Sarangi J et al. Prevention of post splenectomy sepsis: a population based approach. Journal of Public Health Medicine. 1997;19:208–212. doi: 10.1093/oxfordjournals.pubmed.a024611. [DOI] [PubMed] [Google Scholar]
- 9.Working Party of the British Committee for Standards in Haematology Task Force. Guidelines for the prevention and treatment of infection in patients with an absent or dysfunctional spleen. British Medical Journal. 1996;312:430–434. doi: 10.1136/bmj.312.7028.430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Davies JM, Barnes R, Milligan D. Working Party of the British Committee for Standards in Haematology Task Force. Update of guidelines for the prevention and treatment of infection in patients with an absent or dysfunctional spleen. Clinical Medicine. 2002;2:440–443. doi: 10.7861/clinmedicine.2-5-440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Konradsen HB et al. Antibody levels against Streptococcus pneumoniae and Haemophilus influenzae type b in a population of splenectomized individuals with varying vaccination status. Epidemiology and Infection. 1997;119:167–174. doi: 10.1017/s0950268897007978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Siddins M et al. Prophylaxis against postsplenectomy pneumococcal infection. Australia and New Zealand Journal of Surgery. 1990;60:183–187. [PubMed] [Google Scholar]
- 13.MacInnes J, Waghorn DJ, Haworth E. Management of asplenic patients in South Buckinghamshire: an audit of local practice. Communicable Disease Report. CDR Review. 1995;5:R173–R177. [PubMed] [Google Scholar]
- 14.Galan P, Oliva E. Review of infectious prophylaxis in splenectomized patients in the island of Menorca. Medicina Clinica (Barcelona) 2001;117:771–772. doi: 10.1016/s0025-7753(01)72253-1. [DOI] [PubMed] [Google Scholar]
- 15.Kyaw MH et al. A survey of vaccine coverage and antibiotic prophylaxis in splenectomised patients in Scotland. Journal of Clinical Pathology. 2002;55:472–474. doi: 10.1136/jcp.55.6.472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bridgen ML, Pattullo A, Brown G. Pneumococcal vaccine administration associated with splenectomy: the need for improved education, documentation, and the use of a practical checklist. American Journal of Hematology. 2000;65:25–29. doi: 10.1002/1096-8652(200009)65:1<25::aid-ajh4>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 17.Ejstrud P, Hansen JB, Andreasen DA. Prophylaxis against pneumococcal infection after splenectomy: a challenge for hospitals and primary care. European Journal of Surgery. 1997;163:733–738. [PubMed] [Google Scholar]
- 18.Deodhar HA, Marshall RJ, Barnes JN. Increased risk of sepsis after splenectomy. British Medical Journal. 1993;307:1408–1409. doi: 10.1136/bmj.307.6916.1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kind EA et al. Pneumococcal vaccine administration associated with splenectomy: missed opportunities. American Journal of Infection Control. 1998;26:418–422. doi: 10.1016/s0196-6553(98)70038-0. [DOI] [PubMed] [Google Scholar]
- 20.Kinnersley P, Wilkinson CE, Srinivasan J. Pneumococcal vaccination after splenectomy: survey of hospital and primary care records. British Medical Journal. 1993;307:1398–1399. doi: 10.1136/bmj.307.6916.1398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Deodhar M, Kakkar N. An audit of splenectomies in a teaching hospital in North India. Are postsplenectomy guidelines being complied with? Journal of Clinical Pathology. 2004;57:407–410. doi: 10.1136/jcp.2003.013896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.de Montalembert M, Lenoir G. Antibiotic prevention of pneumococcal infections in asplenic hosts: admission of insufficiency. Annals of Hematology. 2004;83:18–21. doi: 10.1007/s00277-003-0779-x. [DOI] [PubMed] [Google Scholar]
- 23.Ramachandra J et al. An audit of post-splenectomy prophylaxis – are we following the guidelines? Annals of the Royal College of Surgeons of England. 2003;85:252–255. doi: 10.1308/003588403766274962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gindre S, Ba K, Dellamonica P. Anti-pneumococcal vaccination in splenectomized patients. Evaluation of its use from 1995 to 2000 at the CHU of Nice. Presse Medicale. 2001;30:1592–1593. [PubMed] [Google Scholar]
- 25.Spelman D. Prevention of overwhelming sepsis in asplenic patients: could do better. Lancet. 2001;357:2072. doi: 10.1016/S0140-6736(00)05227-2. [DOI] [PubMed] [Google Scholar]
- 26.Gudiol F. Prevention of fulminant sepsis in splenectomized patients: we keep forgetting health education. Medicina Clinica (Barcelona) 2001;117:776–777. doi: 10.1016/s0025-7753(01)72256-7. [DOI] [PubMed] [Google Scholar]
- 27.Cohen R. Antipneumococcal vaccines in sickle-cell anaemia and asplenia. Presse Medicale. 2003;32:S12–S14. [PubMed] [Google Scholar]