SUMMARY
IgM- and IgG-capture ELISAs are widely used as diagnostic tests for confirmation of dengue virus infection. The positive rate of anti-dengue IgM and IgG detection was examined in primary and secondary dengue virus infections in the setting of a provincial hospital using IgM- and IgG-capture ELISAs. Disease day 1 was defined as the day of onset of symptoms. In total, 232 plasma samples were collected from 106 confirmed dengue cases consisting of 12 primary and 94 secondary infections. In primary infection, anti-dengue IgM was detected in 4 out of 5 samples collected on disease day 5 and in all the 21 samples collected on disease day 6 or later. Specific IgG was detected in 2 out of 5 samples collected on day 12, and in 5 out of 6 samples collected on disease days 13–15, but was not detected in samples collected on disease day 10 or earlier. In secondary infection, IgM was not detected in the samples on disease days 2 and 3, but detected in 20 out of 79 samples collected on days 4–6, in 44 out of 65 on disease days 7–11 and in 40 out of 51 samples on disease days 12–14. In contrast, specific IgG was detected in 21 out of 60 samples on disease days 4 and 5, in 13 out of 19 on disease day 6, in 62 out of 65 on disease days 7–11 and in all the samples collected on disease day 12 or later. The result indicate that seroconversion rates of IgM and IgG are different between primary and secondary infections, and suggest that detection of specific IgM and IgG is necessary for determining dengue virus infection and for differentiating primary and secondary dengue infections.
INTRODUCTION
Dengue fever (DF) and dengue haemorrhagic fever (DHF) are serious illnesses in many tropical and subtropical countries including Thailand. The causative agents are dengue viruses which belong to the family Flaviviridae, genus Flavivirus. There are four dengue viruses: dengue virus types 1, 2, 3 and 4. The first Thai dengue case was reported in Bangkok in 1958, and thereafter dengue cases were reported in provinces close to Bangkok. The highest number of dengue cases was reported in 1987: 174 285 cases with a morbidity rate of 325/100 000 population. In 2002, the reported number of dengue cases was 114 800 with a morbidity rate of 183/100 000 population. Clinical observation is important for diagnosis of DF and DHF, and virological and serological tests are essential for confirmation of dengue virus infection. Virus isolation is the definitive test, but it is time consuming [1–4]. Reverse transcriptase polymerase chain reaction (RT–PCR) has been used for dengue diagnosis and serotyping [5–9]. The haemagglutination inhibition (HI) test has been widely used as the main serological test; however, it requires paired sera and has cross-reactivity with other flaviviruses [10]. Antibody-capture enzyme-linked immunosorbent assay (ELISA) has been recently used instead of the HI test in many laboratories [11–16]. It has been reported that detection of IgM by antibody-capture ELISA is a reliable diagnostic test both in primary and secondary dengue infections. In the present study, we examined the sensitivity of IgM- and IgG-capture ELISAs for detecting primary and secondary dengue virus infections in the setting of a provincial hospital.
METHODS
Specimen collection and definitions
Peripheral blood specimens were collected for diagnostic purpose from 106 confirmed dengue patients, who were hospitalized in Paediatric Ward II of Sawanpracharak Hospital, Nakhon Sawan province, Thailand from May to September 2002. The first blood samples were collected on the admission day and the second samples were collected on the discharge day or 7 days after the first sample. When the interval between the first and second samples was <7 days, a third sample were collected. The third samples were collected at least 7 days after the first blood collection. Plasma samples were separated from peripheral blood specimens at the Sawanpracharak Hospital. One vial each of the plasma samples was kept in a liquid nitrogen tank and the other vials of the same samples were kept at −20°C. All the plasma samples were transported in dry ice to the Arbovirus Laboratory, National Institute of Health (Thai NIH), Department of Medical Sciences, Nonthaburi, Thailand. A total of 232 plasma samples from the 106 cases were used in the analysis. The total of 106 cases consisted of 55 females and 51 males. The average age of the cases was 9·2 years (range 4–14 years). The first plasma specimens from the 106 cases were examined for dengue viral genome by RT–PCR. Dengue virus type 1 was detected in 29 specimens, type 2 in 50 specimens, type 3 in 10 specimens and type 4 in 9 specimens. In the present study, disease day 1 was defined as the day of onset of symptoms [9, 17].
Antibody-capture ELISA
IgM and IgG antibody-capture ELISAs were performed as previously described by Innis et al. [12] with some modifications. In brief, 96-well U-bottomed microtitre plates (Maxisorp, Nunc, Denmark) were coated with 100 μl of anti-human IgM or anti-human IgG (anti-mu or anti-gamma chain) (Capple, Aurora, OH, USA) diluted in carbonate-bicarbonate buffer. The plates were incubated at 4°C overnight. After washing six times with PBS-T, diluted samples, strong positive control, weak positive control or negative control, were added in duplicate and the plates were incubated at room temperature for 2 h. The plates were washed as described above. Pooled dengue viral antigen was added, and the plates were incubated at room temperature for 2 h. After washing, 25 μl of horseradish peroxidase (HRP)-human anti-flavivirus IgG conjugate was added and the plates were incubated at 37°C for 1 h. After washing, 100 μl of o-phenylenediamine dihydrochloride (OPD; Sigma, St. Louis, MO, USA) substrate containing 3·3 μl/ml of 3% H2O2 was added. The plates were incubated in the dark at room temperature for 15–30 min. The colour development was stopped by addition of 50 μl of 4 m H2SO4 per well and OD was read at 490 nm using a microplate autoreader (Model EL 311; Bio-tex Instruments, Winooski, VT, USA). The OD of the results was transformed to binding index (BI) units. Anti-dengue virus IgM was determined to be positive when the BI is 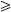 40 units, and anti-dengue virus IgG was determined to be positive when
40 units, and anti-dengue virus IgG was determined to be positive when 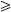 80 units. The cases were diagnosed as primary infection when the IgM:IgG ratio was
80 units. The cases were diagnosed as primary infection when the IgM:IgG ratio was 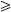 1·8, and as secondary infection when the ratio was <1·8.
1·8, and as secondary infection when the ratio was <1·8.
HI test
The HI test was performed as previously reported [10, 18]. Briefly, the antigen was diluted with 0·4% bovine albumin serum in borate saline buffer (BABS) to make 8 haemagglutination (HA) units/50 μl in veronal adjusting diluent (VAD). The natural non-specific inhibitors in the plasma were removed by mixing plasma with kaolin-borate saline buffer. Thereafter, natural haemagglutinins were adsorbed by packed goose red blood cells (GRBC). The treated plasma samples were serially diluted two-fold from 1:10 to 1:10 240 with 0·4% BABS in U-bottomed microtitre plates. The positive and negative plasma controls were included in the test and they were treated and diluted in the same way as tested plasma. Subsequently, 25 μl of dengue viral antigen was added to each well containing 25 μl of diluted plasma and the plates were shaken thoroughly. The plates were incubated at 4°C overnight, and 50 μl of 0·33% GRBC in proper VAD was added to each well. The plates were incubated at 37°C for 1 h. HI titres on the tested plates were read. The case was determined to be primary infection when HI titre demonstrated a four-fold or greater increase between paired samples and convalescent HI titre was <2560. The case was determined to be secondary infection when HI titre was 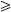 2560 [19].
2560 [19].
RT–PCR
RT–PCR was performed as previously reported [18]. Briefly, RNA was extracted from 100 μl of plasma using QIAamp viral RNA mini kit. RT and PCR were done in one tube using universal primer and a one-step RT kit (Qiagen GmbH, Hilden, Germany). The reaction tube was placed in a thermal cycler machine (PerkinElmer-Cetus, Norwalk, CT, USA). The primary PCR product was further used for nested PCR in another reaction tube. The nested PCR reaction was performed in one tube, containing four specific primer pairs of dengue types 1, 2, 3 and 4, using a thermal cycler. The secondary PCR product was subjected to agarose gel electrophoresis with dengue virus markers. Amplified DNA fragments were visualized by ethidium bromide staining. The expected product sizes of dengue virus types 1, 2, 3 and 4 were 504, 346, 196 and 143 bp respectively.
RESULTS
Of the 118 originally enrolled dengue cases, 12 cases were determined to be primary infection and 96 to be secondary, on the basis of the results of HI test and ELISA. Ten cases could not be determined to be either primary or secondary. Twenty-seven and 205 plasma samples from 12 primary and 94 secondary dengue virus infection cases respectively, were used in the present analysis. We analysed data based on serological diagnosis, in primary dengue virus infection.
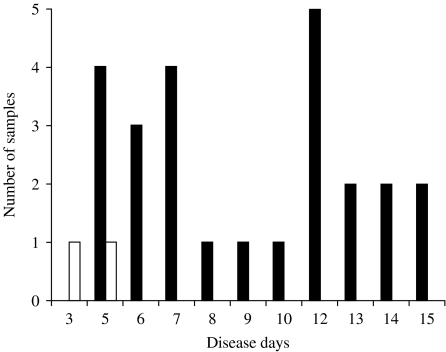
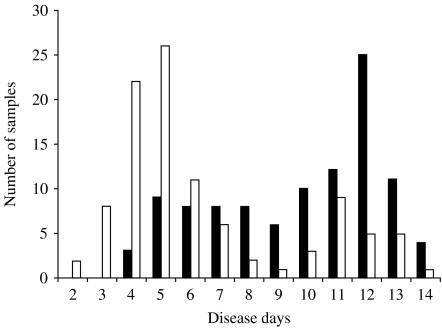
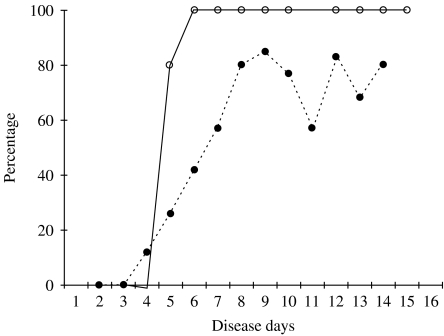
IgM responses were first analysed. In primary dengue virus infection, anti-dengue IgM was detected in 25 out of 27 plasma samples. When the data were analysed based on disease day, specific IgM was detected in four out of five samples collected on disease day 5 and in all the samples collected on disease day 6 or later (Fig. 1). In secondary infection, specific IgM was not detected in any of 10 samples collected on disease days 2 and 3, but detected in 20 out of 79 samples on disease days 4–6, in 44 out of 65 on disease days 7–11, and in 40 out of 51 samples on disease days 12–14 (Fig. 2).
Fig. 1.
Detection of anti-dengue virus IgM by IgM-capture ELISA in plasma samples collected from primary dengue virus infection cases. □, Negative; ■, positive.
Fig. 2.
Detection of anti-dengue virus IgM by IgM-capture ELISA in plasma samples collected from secondary dengue virus infection cases. □, Negative; ■, positive.
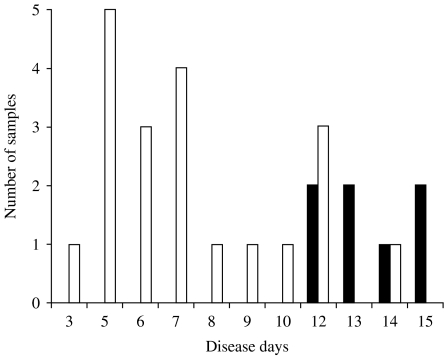
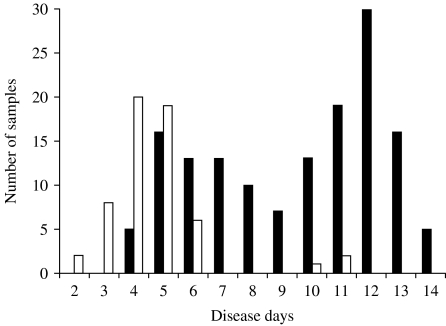
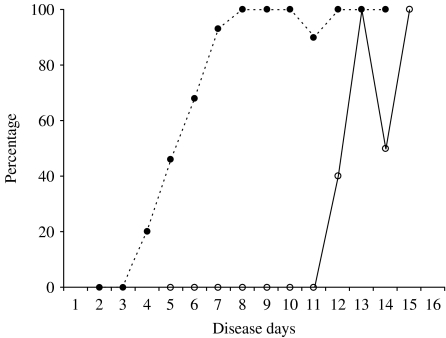
IgG responses were next analysed. In primary infection, anti-dengue IgG was not detected in any sample collected on disease day 10 or earlier, but was detected in two out of five samples on disease day 12, and in five out of six samples collected on disease days 13–15 (Fig. 3). In secondary infection, anti-dengue IgG was not detected in any samples collected on disease days 2 and 3, but was detected in 21 out of 60 samples on disease days 4 and 5, in 13 out of 19 samples on disease day 6, in 62 out of 65 samples on disease days 7–11 and in all the samples collected on disease day 12 or later (Fig. 4).
Fig. 3.
Detection of anti-dengue virus IgG by IgG-capture ELISA in plasma samples collected from primary dengue virus infection cases. □, Negative; ■, positive.
Fig. 4.
Detection of anti-dengue virus IgG by IgG-capture ELISA in plasma samples collected from secondary dengue virus infection cases. □, Negative; ■, positive.
When the IgM-positive rate was compared, it was higher in primary infection than in secondary infection on disease day 5 or later (Fig. 5). In contrast, the IgG-positive rate was higher in secondary infection than in primary infection on disease days 4–12 (Fig. 6).
Fig. 5.
The percentage of IgM-positive samples in primary and secondary dengue virus infections. ○, primary infection; •, secondary infection.
Fig. 6.
The percentage of IgG-positive samples in primary and secondary dengue virus infections. ○, primary infection; •, secondary infection.
DISCUSSION
IgM-capture ELISA has been widely used for laboratory diagnosis of dengue virus infection and is considered to be a reliable serological test [9, 12, 16, 17, 20, 21]. In the present study, we attempted to detect anti-dengue virus IgM and IgG by antibody-capture ELISA in plasma samples collected from dengue patients at various days of illness in the setting of a provincial hospital in Thailand. Anti-dengue IgM antibody was detected in four out of five plasma samples collected on disease day 5 and all the 21 samples collected on disease day 6 or later in primary cases. IgM was first detected in samples from secondary cases on disease day 4. Its positive rate was >50%, when samples were collected on disease day 7 or later. Anti-dengue IgG was detected in most of the samples collected from secondary infection cases on day 7 or later. Of 113 samples collected on disease days 7–14, 116 samples were IgG positive, and only three were negative. Plasma or serum samples collected at a very early stage are not suitable for IgM detection. However, after disease day 5 in primary infection and day 6 in secondary infection, IgM and IgG were positive in most of the samples. Thus, selection of sample collection date is important for antibody-capture ELISA. The results in the present study confirmed the previous finding that IgM responses in secondary dengue virus infection are not as high as in primary infection, whereas the anti-dengue IgG-positive rate is higher in secondary than in primary cases especially in early stage of the illness [17, 20, 22]. Our results also support the idea that detection of both specific IgM and IgG is important for confirmation of dengue virus infection. There have been reports that RT–PCR is also a reliable test in both primary and secondary dengue virus infections when samples are collected at a febrile stage [9, 18]. Therefore, the combination of antibody-capture ELISA and RT–PCR can increase the accuracy and the sensitivity of laboratory diagnosis of dengue virus infection.
ACKNOWLEDGEMENTS
We thank Dr Somchai Sangkitporn, Ms. Suntharee Rojanasuphot, and staff of the Arbovirus Laboratory at Thai NIH for their help and support. We also thank the doctors, nurses, and laboratory staff at Sawanpracharak Hospital and the staff at the Nakhon Sawan provincial health office for their support and cooperation. This study was supported by a grant from Japan Health Science Foundation.
DECLARATION OF INTEREST
None.
REFERENCES
- 1.Russell PK et al. A plaque reduction test for dengue virus neutralization antibodies. Journal of Immunology. 1967;99:291–296. [PubMed] [Google Scholar]
- 2.Yuill TM et al. Dengue-virus recovery by direct and delayed plaque in LLC-MK2 cells. American Journal of Tropical Medicine and Hygiene. 1968;17:441–448. doi: 10.4269/ajtmh.1968.17.441. [DOI] [PubMed] [Google Scholar]
- 3.Rosen L, Gubler DJ. The use of mosquitoes to detect and propagate dengue viruses. American Journal of Tropical Medicine and Hygiene. 1974;23:1153–1160. doi: 10.4269/ajtmh.1974.23.1153. [DOI] [PubMed] [Google Scholar]
- 4.Henchal EA et al. Rapid identification of dengue virus isolations by using monoclonal antibodies in an indirect immunofluorescence assay. American Journal of Tropical Medicine and Hygiene. 1983;32:164–169. doi: 10.4269/ajtmh.1983.32.164. [DOI] [PubMed] [Google Scholar]
- 5.Morita K, Tanaka M, Igarashi A. Rapid identification of dengue virus serotypes by using polymerase chain reaction. Journal of Clinical Microbiology. 1991;29:2107–2110. doi: 10.1128/jcm.29.10.2107-2110.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lanciotti RS et al. Rapid detection and typing of dengue viruses from clinical samples by using reverse transcriptase-polymerase chain reaction. Journal of Clinical Microbiology. 1992;30:545–551. doi: 10.1128/jcm.30.3.545-551.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Maneekan N et al. Applications of polymerase chain reaction for identification of dengue viruses isolationfrom patient sera. Microbiology and Immunology. 1993;37:41–47. doi: 10.1111/j.1348-0421.1993.tb03177.x. [DOI] [PubMed] [Google Scholar]
- 8.Yenchitsomanus P et al. Rapid detection and identification of dengue viruses by polymerase chain reaction (PCR) Southeast Asian Journal of Tropical Medicine and Public Health. 1996;27:228–236. [PubMed] [Google Scholar]
- 9.Yamada K et al. Laboratory diagnosis of dengue virus infections by reverse transcriptase-polymerase chain reaction (RT–PCR) and IgM-capture enzyme-linked immunosorbent assay (ELISA) Japanese Journal of Infectious Diseases. 1999;52:150–155. [PubMed] [Google Scholar]
- 10.Clarke DH, Casal J. Techniques for hemagglutination and hemagglutination-inhibition with arthropod borne virus. American Journal of Tropical Medicine and Hygiene. 1985;7:561–573. doi: 10.4269/ajtmh.1958.7.561. [DOI] [PubMed] [Google Scholar]
- 11.Bundo K, Igarashi A. Antibody-capture ELISA for detection of immunoglobulin M antibodies in sera from Japanese encephalitis and dengue hemorrhagic fever patients. Journal of Virological Methods. 1985;11:15–22. doi: 10.1016/0166-0934(85)90120-x. [DOI] [PubMed] [Google Scholar]
- 12.Innis BL et al. An enzyme-linked immunosorbent assay to characterize dengue infections where dengue and Japanese encephalitis co-circulate. American Journal of Tropical Medicine and Hygiene. 1989;40:418–427. doi: 10.4269/ajtmh.1989.40.418. [DOI] [PubMed] [Google Scholar]
- 13.Kuno G, Gomez I, Gubler DJ. An ELISA procedure for diagnosis of dengue infections. Journal of Virological Methods. 1991;33:101–113. doi: 10.1016/0166-0934(91)90011-n. [DOI] [PubMed] [Google Scholar]
- 14.Cardosa MJ, Zuraini I. Comparison of IgM capture ELISA with a dot enzyme immunoassay for laboratory diagnosis of dengue virus infections. Southeast Asian Journal of Tropical Medicine and Public Health. 1991;22:337–340. [PubMed] [Google Scholar]
- 15.Tio PH, Malasit P. Anti-dengue IgG detection by an indirect ELISA. Southeast Asian Journal of Tropical Medicine and Public Health. 1995;26:673–676. [PubMed] [Google Scholar]
- 16.Nawa M et al. Serotype-cross-reactive IgM responses in dengue virus infections determined by enzyme-linked immunosorbent assays. Clinical and Diagnostic Laboratory Immunology. 2000;7:774–777. doi: 10.1128/cdli.7.5.774-777.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vaughn DW et al. Dengue in the early febrile phase: viremia and antibody responses. Journal of Infectious Disease. 1997;176:322–330. doi: 10.1086/514048. [DOI] [PubMed] [Google Scholar]
- 18.Sa-Ngasang A et al. Evaluation of RT–PCR as a tool for diagnosis of secondary dengue virus infection. Japanese Journal of Infectious Diseases. 2003;56:205–209. [PubMed] [Google Scholar]
- 19.WHO. Dengue Hemorrhagic Fever: Diagnosis, Treatment, Prevention and Control. 2nd edn. Geneva: World Health Organization; 1997. [Google Scholar]
- 20.Chanama S et al. Analysis of specific IgM response in secondary dengue infection: level and positive rates in comparison with primary infection. Journal of Clinical Virology. 2004;31:185–189. doi: 10.1016/j.jcv.2004.03.005. [DOI] [PubMed] [Google Scholar]
- 21.Palmer CJ et al. Evaluation of the MRL diagnostics dengue fever IgM capture ELISA and the PanBio Rapid Immunochromatographic Test for diagnosis of dengue fever in Jamaica. Journal of Clinical Microbiology. 1999;37:1600–1601. doi: 10.1128/jcm.37.5.1600-1601.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Innis BL., Gubler DJ, Kuno G. Dengue and Dengue Hemorrhagic Fever. New York: CAB International; 1997. Antibody responses to dengue virus infection; pp. 221–245. : pp. [Google Scholar]