SUMMARY
From 2000 to 2001, nine strains of Salmonella enterica belonging to the new serotype Keurmassar have been isolated from human and poultry samples at the Senegalese National Salmonella and Shigella Reference Laboratory at the Pasteur Institute, in Dakar. All strains carried virulence factors including Salmonella Pathogenicity Islands (SPI)-1, -2, -3 and -5 encoded genes. Strains did not harbour virulence plasmid. Ribotyping analysis revealed a single clone identical to Salmonella Decatur isolated in Zimbabwe. These data suggest that strains are closely related, and may have been spread clonally. In this new serotype, insertion sequence IS200 is not present.
INTRODUCTION
Salmonella enterica is one of the most common causes of foodborne enteric infection in humans and the main source of infection is contaminated food of animal origin. According to estimations of the World Health Organization, there are annually 16·6 million cases of typhoid fever, with approximately 600 000 fatal outcomes, and 1·3 billion cases of acute gastroenteritis due to non-typhoidal salmonellosis, with 3 million deaths [1]. In developing countries, few data are available on the incidence of infections due to Salmonella. In these areas, only 1–10% of cases are reported, and where the disease is more severe, it is associated with 20–30% mortality [1].
In 2000–2001, the Senegalese National Salmonella and Shigella Reference Laboratory at the Pasteur Institute in Dakar received 604 Salmonella samples; among them, a new serotype of Salmonella (Salmonella enterica serotype 35:c:1,2) named Keurmassar was identified. This new serotype was isolated from human and poultry samples [2]. Multiple virulence factors are involved in the pathogenesis of Salmonella. These virulence genes act together in a complex virulence function, and have been found on horizontally acquired DNA regions, such as pathogenicity islands, plasmids, transposons, and bacteriophages [3, 4].
Herein, we report for the first time in Africa, the characterization of virulence factors in the newly described S. enterica serotype Keurmassar.
MATERIALS AND METHODS
Nine S. enterica serotype Keurmassar strains were isolated in Senegal from human and poultry samples between 2000 to 2001 in the Senegalese National Salmonella and Shigella Reference Laboratory at the Pasteur Institute, Dakar.
Total genomic DNA was extracted as described previously [5]. Crude extracts for polymerase chain reaction (PCR) assays were obtained by the boiling method as described by Holmes [6]. The following molecular methods were used to type strains:
For ribotyping analysis, ∼2 μg of extracted DNA were digested overnight at 37°C with PvuII. Restriction fragments were separated by electrophoresis in a 0·8% (w/v) agarose gel in Tris–acetate buffer for 16 h at 1·5 V/cm and transferred to Hybond N membranes by the method of Southern [7]. Restriction fragments of lambda DNA cleaved with HindIII were used as fragment size markers.
Analysis of IS200 content was performed by PCR using S. enterica serovar Typhimurium strain ATCC 14028s and S. enterica serotype Abortusovis strain SS44 as positive controls. The following primers (5′-CAG ATG CGC CTA TAA GGC T-3′ and 5′-CTA GGC TGG GGG TTC CGG GAA-3′) were used to amplify a fragment of 690 bp [8].
Virulence factors encoded within Salmonella Pathogenicity Islands (SPIs) were detected by PCR using the following primers: invA (5′-TGC CTA CAA GCA TGA AAT GG-3′ and 5′-AAA CTG GAC CAC GGT TGA CAA-3′), spiC (5′-CCT GGA TAA TGA CTA TTG AT-3′ and 5′-AGT TTA TGG TGA TTG CGT AT-3′), misL (5′-GTC GGC GAA TGC CGC GAA TA-3′ and 5′-GCG CTG TTA ACG CTA ATA GT-3′), orfL (5′-GGA GTA TCG ATA AAG ATG TT-3′ and 5′-GCG CGT AAC GTC AGA ATC AA-3′), pipD (5′-CGG CGA TTC ATG ACT TTG AT-3′ and 5′-CGT TAT CAT TCG GAT CGT AA-3′), spvR (5′-CCC CGG GAA TTC GCT GCA TAA GGT AGA AGG-3′ and 5′-CCC CGG GTA CCA TGG ATT TCT TGA TTA ATA AA-3′). Detection of Gifsy-1 and Gifsy-2 phages was performed as previously described [3]. S. Typhimurium strain ATCC 14028s was used as positive control.
RESULTS AND DISCUSSION
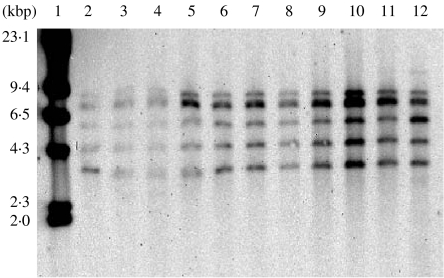
The nine strains of S. Keurmassar isolated from human and poultry specimens showed identical ribotype patterns suggesting that all strains isolated between 2000 to 2001 may belong to the same clone (Fig.). Unexpectedly, this ribotype was identical to the most frequently occurring profile observed in strains of S. enterica serotype Decatur [9]. Interestingly, strains of S. Decatur (6,7:c:1,5) have been rarely isolated worldwide, yet a number strains belonging to this serotype have recently been isolated in Zimbabwe (Rubino et al., unpublished observations).
Fig.
Ribotype patterns obtained from Salmonella enterica subsp. enterica serotype Keurmassar. Lane 1, Lambda molecular weight ladder (kbp); lanes 2–11, S. Keurmassar (strains LO4020128, LO4020129, LO4020157, LO4020157, KO330119, KO412155, KO405195, KO329122, KO509194, KO508153); lane 12, S. Decatur, strain 1623.
The screening by PCR to detect IS200 was negative for all strains. This insertion sequence is present in most isolates of Salmonella and certain strains of Shigella and Escherichia coli [10, 11]. These data would suggest that S. Keurmassar belongs to a small group of non-typhoid S. enterica subspecies enterica, including serotypes Agona, Paratyphi C, Choleraesuis, and Typhisuis, that lack genomic copies of IS200 [9–11].
All isolates investigated for the presence of virulence factors revealed the presence of invA, spiC, misL, pipD, contained respectively in SPI-1, SPI-2, SPI-3, and SPI-5. These SPIs are required for bacterial penetration in the epithelial cells of intestine and for growth and survival of bacteria in the host [12]. Pathogenicity Islands (PAIs) have also been described in uropathogenic [13], and enteropathogenic E. coli [14].
SPI-4 gene orfL was absent in all strains; SPI-4 is involved in secretion of toxins that induce apoptosis in immune cells and it is required for survival in macrophages [15].
None of the S. Keurmassar strains harbour a virulence plasmid. A cluster of five genes, the spv locus, accounts for the overall contribution of virulence plasmids to pathogenicity and systemic infection in animal models: spvR a positive regulator gene and four effector genes, spvA, spvB, spvC and spvD [16]. These virulence genes are found in the most frequently isolated non-typhoid serotypes of S. enterica subsp. enterica including serotypes Typhimurium, Enteritidis, Dublin, Choleraesuis, Gallinarum, and Abortusovis and in S. enterica subsp. houtenae [17].
All isolates of S. Keurmassar were non-lysogenic for Gifsy-1 and Gifsy-2, two prophages that carry several virulence genes. Gifsy-2 genome includes the sodC-1 gene coding for a Cu Zn-dependent periplasmic superoxide dismutase; Gifsy-2 also encodes a virulence effector, SseI, secreted by the SPI-2 type III secretion system. The virulence of Gifsy-1 is undetectable in the presence of Gifsy-2; it becomes significant in cells that lack Gifsy-2, but carry the sodC-1 gene [18]. In our study, the sodC-1 gene was absent (data not shown).
Overall, genetic characterization of S. Keurmassar strains revealed the lack (i.e. Gifsy1, Gifsy2, spv) and/or the partial deletion (i.e. SPI-4) of several genetic loci previously known to play an important contribution to S. enterica pathogenicity. However, S. Keurmassar strains seem to be quite virulent to successfully establish Salmonella-induced enteritis in man.
In Africa, to our knowledge, this is the first report of the characterization of virulence factors in Salmonella.
ACKNOWLEDGEMENTS
This work was supported by a grant from the Sardinian Regional Government to S.U. (grant L.R. 19/96).
DECLARATION OF INTEREST
None.
REFERENCES
- 1.Pang T et al. Typhoid fever and other salmonellosis: a continuing challenge. Trends in Microbiology. 1995;3:253–255. doi: 10.1016/s0966-842x(00)88937-4. [DOI] [PubMed] [Google Scholar]
- 2.Cardinale E et al. Dual emergence in food and human of a novel multiresistant serotype of Salmonella in Senegal: Salmonella enterica subsp. enterica serotype 35:c:1,2. Journal of Clinical Microbiology. 2001;39:S2373–S2374. doi: 10.1128/JCM.39.6.2373-2374.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bacciu D et al. Transposition of heat-stable toxin astA gene into a Gifsy2-related prophage of Salmonella enterica serovar Abortusovis. Journal of Bacteriology. 2004;186:4568–4574. doi: 10.1128/JB.186.14.4568-4574.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Häcker J et al. Pathogenicity islands of virulent bacteria: structure, function and impact on microbial evolution. Molecular Microbiology. 1997;23:1089–1097. doi: 10.1046/j.1365-2958.1997.3101672.x. [DOI] [PubMed] [Google Scholar]
- 5.Wilson K, Ausubel FA. Current Protocols in Molecular Biology. New York: John Wiley & Sons.; 1994. Preparation of genomic DNA from bacteria; pp. 2.4.1–2.4.5. : pp. [DOI] [PubMed] [Google Scholar]
- 6.Holmes DS, Quigley M. A rapid boiling method for the preparation of bacterial plasmid. Analytical Biochemistry. 1981;114:193–197. doi: 10.1016/0003-2697(81)90473-5. [DOI] [PubMed] [Google Scholar]
- 7.Southern EM. Detection of specific sequences among DNA fragments separated by gel electrophoresis. Journal of Molecular Biology. 1975;98:503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- 8.Bisercic M, Ochman N. Natural populations of Escherichia coli and Salmonella Typhimurium harbor the same classes of insertion sequences. Genetics. 1993;133:449–453. doi: 10.1093/genetics/133.3.449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Uzzau S, Hovi M, Stocker BA. Application of ribotyping and IS200 fingerprinting to distinguish the five Salmonella serotype O:6,7:c:1,5 groups: Choleraesuis sensu stricto, Choleraesuis var. Kunzendorf, Choleraesuis var. Decatur, Paratyphi C, and Typhisuis. Epidemiology and Infection. 1999;123:37–46. doi: 10.1017/s0950268899002654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gibert I, Barbé J, Casadesús J. Distribution of insertion sequence IS200 in Salmonella and Shigella. Journal of General Microbiology. 1990;136:2555–2560. doi: 10.1099/00221287-136-12-2555. [DOI] [PubMed] [Google Scholar]
- 11.Bisercic M, Ochman N. The ancestry of insertion sequences common to Escherichia coli and Salmonella Typhimurium. Journal of Bacteriology. 1993;175:7863–7868. doi: 10.1128/jb.175.24.7863-7868.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Marcus S et al. Salmonella pathogenicity islands: big virulence in small packages. Microbes and Infection. 2000;2:145–156. doi: 10.1016/s1286-4579(00)00273-2. [DOI] [PubMed] [Google Scholar]
- 13.Blum G et al. Excision of large DNA regions termed pathogenicity islands from t-RNA-specific loci in the chromosome of an Escherichia coli wild-type pathogen. Infection and Immunity. 1994;62:606–614. doi: 10.1128/iai.62.2.606-614.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McDaniel T et al. A genetic locus of enterocyte effacement conserved among diverse enterobacterial pathogens. Proceedings of the National Academy of Sciences USA. 1995;92:1664–1668. doi: 10.1073/pnas.92.5.1664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schmidt H, Hensel M. Pathogenicity Islands in bacterial pathogenesis. Clinical Microbiology Reviews. 2004;17:14–56. doi: 10.1128/CMR.17.1.14-56.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gulig P et al. Molecular analysis of spv virulence genes of the Salmonella virulence plasmids. Molecular Microbiology. 1993;7:825–830. doi: 10.1111/j.1365-2958.1993.tb01172.x. [DOI] [PubMed] [Google Scholar]
- 17.Aabo S, Brown DJ, Olsen JE. Virulence characterization of a strain of Salmonella enterica subspecies houten (subspecies IV) with chromosomal integrated Salmonella plasmid virulence (spv) genes. Research in Microbiology. 2000;151:183–189. doi: 10.1016/s0923-2508(00)00138-8. [DOI] [PubMed] [Google Scholar]
- 18.Figueroa-Bossi N, Bossi L. Inducible prophages contribute to Salmonella virulence in mice. Molecular Microbiology. 1999;33:167–176. doi: 10.1046/j.1365-2958.1999.01461.x. [DOI] [PubMed] [Google Scholar]