SUMMARY
From January 1997 to December 2003, all patients with non-tuberculous mycobacteria (NTM) isolation who were treated at a university hospital in Taiwan were evaluated. Among the 2650 NTM isolates, 1225 (46·2%) were from 412 patients with clinically significant diseases. The annual incidence (per 100 000 patients) of disease caused by NTM was 8·96 in 1997, 21·53 in 2002, and 16·55 in 2003. The major types of infections caused by NTM included isolated pulmonary infection and pleurisy (59·5%), skin/soft-tissue infections and osteomyelitis (13·8%), and disseminated diseases (13·3%). The two most common groups of organisms involved were rapidly growing mycobacteria (RGM) (41·4%) and Mycobacterium avium complex (MAC) (39%). The most common organism involved in isolated pulmonary infection and pleurisy was MAC (44·1%). RGM predominated in keratitis (94%), skin/soft-tissue infections and osteomyelitis (43·9%), and lymphadenitis (66·7%). This retrospective 7-year study demonstrated an increase in the incidence of NTM disease in a university hospital.
INTRODUCTION
Non-tuberculous mycobacteria (NTM) have been well recognized as a cause of human disease since the early 1950s [1]. These organisms are ubiquitous in the environment, readily isolated from water, soil, domestic and wild animals, milk, and foods [2–4]. They are not always pathogenic when isolated from human samples, and the identification of which isolates are pathogenic, contaminants or colonizers is based on internationally recognized criteria [4, 5]. These organisms have been implicated in an increasing number of human diseases around the world. This problem has become particularly prominent in patients with impaired cell-mediated immunity [e.g. due to immunosuppressive medication, acquired immunodeficiency syndrome (AIDS), or a genetic deficiency in the pathway to macrophage activation] [6]. Most immunocompetent individuals can effectively resist infection by these organisms without tissue invasion occurring. The rise in clinical significance has been related to the advent of improved techniques in clinical mycobacteriology, greater awareness of the pathogenic nature of these organisms among physician, and the increased population of immunocompromised hosts [4, 5].
Infections due to NTM have been reported to account for 0·5–30% of all mycobacterial infections [2, 4, 5]. Recent studies have indicated that disease caused by NTM is on the rise, and in many developed countries NTM account for an increasing proportion of mycobacterial disease. The distribution of various NTM species is not uniform and is geographically and environmentally dependent. The clinical significance and disease spectrum of NTM in Taiwan has been previously reported by Shih et al. for the period 1992–1996 [7]. The criteria for diagnosis of disease caused by NTM had been revised in the 1997 American Thoracic Society (ATS) Guidelines, which adopted stricter bacteriological, radiographic, and clinical criteria [5]. The purpose of this study was to investigate the NTM isolates in a university hospital in Taiwan from 1997 to 2003 in order to elucidate the changing epidemiology and disease spectrum caused by these organisms.
MATERIALS AND METHODS
Isolates of NTM
We retrospectively reviewed the records from the Mycobacteriology Laboratory of National Taiwan University Hospital from January 1997 to December 2003. Preparation of different clinical specimens for cultures of mycobacteria followed the recommended guidelines [7, 8]. The concentrated specimen was inoculated onto the Lowenstein–Jensen slants and Middlebrook 7H11 medium (BBL; Becton Dickinson Diagnostic Instrument Systems, Sparks, MD, USA) prior to 2000 and onto both Lowenstein–Jensen slants and the fluorometric BACTEC technique (BACTEC MGIT 960 system, Becton Dickinson) since 2000. NTM were identified to species level using conventional biochemical methods [8]. Some isolates, particularly those associated with clinical disease in this study were further confirmed by sequencing of their 16S rRNA gene (1464 bp) using the two primers (8FPL and 1492) as previously described [9].
Definition of NTM disease
Pulmonary disease due to NTM was diagnosed if the clinical, radiological, and microbiological characteristics of patients met the criteria established in 1997 by the ATS for non-tuberculous mycobacterial lung disease [5]. Pleurisy due to NTM was diagnosed if NTM was isolated from a pleural effusion specimen but not from other body sites. Disseminated disease was diagnosed if the NTM isolate was recovered from more than one body site or from blood or bone marrow. Lymphadenitis due to NTM was diagnosed if culture of a biopsy specimen or discharge of a lymph node yielded an NTM isolates. Skin/soft-tissue disease or osteomyelitis was diagnosed if culture of a wound discharge or biopsy specimen of a lesion involving skin, subcutaneous tissue, muscle, synovium, or bone yielded a NTM isolate.
Incidence of disease caused by NTM
The annual incidence of diseases caused by NTM was calculated as the annual number of patients with NTM disease divided by the total number of patients who visited the National Taiwan University Hospital (NTUH), including outpatients and in-patients, in each indicated year. The annual incidence (per 100 000 patients) of diseases caused by Mycobacterium avium complex (MAC) and rapidly growing mycobacteria (RGM) were also determined. RGM consisted of Mycobacterium abscessus, M. fortuitum, M. chelonae, M. phlei, and M. vaccae.
Characteristic of patients
Demographical characteristics, clinical diseases, and underlying medical condition of patients with NTM diseases were reviewed by medical records. The underlying conditions evaluated included: chronic lung disease (CLD), if clinical and radiological findings were consistent or confirmatory findings of respiratory function tests were available; diabetes mellitus; AIDS; malignancy; chronic liver disease (including chronic hepatitis B virus infection, chronic hepatitis C virus infection, alcoholic liver disease, and liver cirrhosis), chronic steroid use (daily use of 20 mg prednisolone for at least 2 weeks), chronic renal disease (serum creatinine level ⩾2 mg/dl), and organ transplantation.
This study was approved by the Research Ethics Committee of the NTUH.
RESULTS
A total of 7442 mycobacterial (M. tuberculosis and NTM) isolates were recovered from patients treated in the NTUH from January 1997 to December 2003. Among these isolates, 2650 (35·6%) were NTM and these were recovered from 1346 patients. The ratio of NTM among all mycobacterial isolates increased gradually from 26·2% in 1997 to 41·8% in 2003. The most common organisms were RGM (41·4%), including M. abscessus (18·3%), M. fortuitum (12·8%), M. chelonae (8·9%), M. phlei (1%), and M. vaccae (0·3%), followed by MAC (39%).
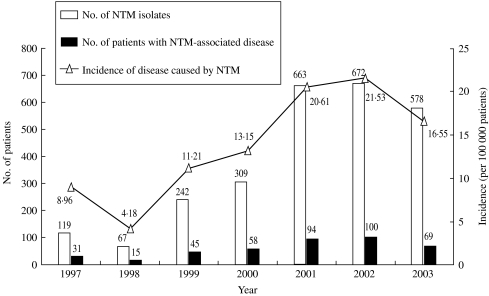
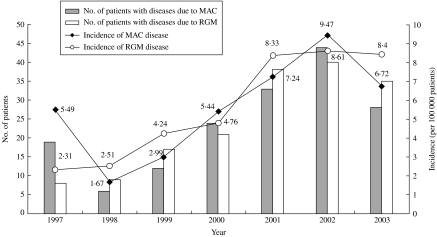
Among the 2650 isolates, 1225 (46·2%) isolates from 412 patients were considered to be associated with the development of clinical diseases. Figure 1 shows the annual number of NTM isolates, annual number of patients with disease caused by NTM, and incidence of NTM-associated disease from 1997 to 2003. The incidence of patients with NTM-associated disease increased from 9·0/100 000 in 1997 to 21·5/100 000 in 2002, but declined in 2003 (16·6/100 000). The two most common pathogens in patients with NTM-associated diseases were MAC and RGM. The incidence of MAC-associated disease increased from 5·5/100 000 patients in 1997 to 9·5/100 000 in 2002, but declined slightly in 2003 (6·7/100 000) (Fig. 2). The incidence of RGM-associated disease was low (2·3/100 000) in 1997, but increased rapidly during the next 6 years (8·6/100 000 in 2002 and 8·4/100 000 in 2003). Among the 1225 NTM isolates, 87·5% were recovered from various respiratory sources.
Fig. 1.
The annual number of non-tuberculous mycobacteria (NTM) isolates and annual number and incidence (per 100 000 patients) of patients with diseases caused by NTM in National Taiwan University Hospital from 1997 to 2003.
Fig. 2.
The annual number and incidence (per 100 000 patients) of patients with diseases caused by M. avium complex (MAC) and rapidly growing mycobacteria (RGM) in National Taiwan University Hospital from 1997 to 2003.
Of these 412 patients with NTM-associated diseases, 245 patients (59·5%) had pulmonary disease or pleurisy, followed by skin/soft-tissue infection and osteomyelitis (13·8%), and disseminated disease (13·3%) (Table 1). The characteristics of patients with these three major NTM-associated diseases are summarized in Table 2. Most patients with pulmonary disease or pleurisy had preexisting lung disease or malignancy. Twelve patients had AIDS. Local factors including trauma and surgery (35·1%) and diabetes mellitus (17·5%) were the most common underlying condition in skin/soft-tissue infection and osteomyelitis. The majority of patients with disseminated disease were male. These patients were younger than those with the other two major diseases associated with NTM (pulmonary disease or pleurisy and skin/soft-tissue infection and osteomyelitis). Forty-one patients (74·5%) had AIDS and one-fifth of these patients had chronic liver disease.
Table 1.
Clinically significant diseases caused by non-tuberculous mycobacteria in National Taiwan University Hospital from 1997 to 2003
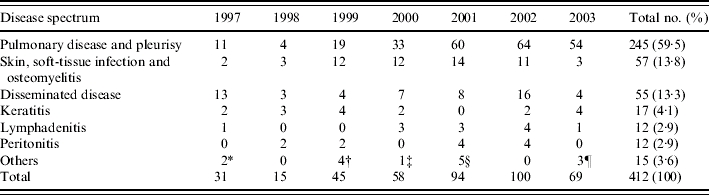
Includes two patients with colitis.
Includes colitis (one patient), spleen abscess (one patient), meningitis (one patient), and urinary tract infection (one patient).
Includes one patient with meningitis.
Includes urinary tract infection (three patients), retroperitoneal abscess (one patient), and meningitis (one patient).
Includes urinary tract infection (two patients) and colitis (one patient).
Table 2.
Demogarphic characteristics of patients with pulmonary disease, skin/soft-tissue infection, and osteomyelitis and disseminated disease caused by non-tuberculous mycobacteria in National Taiwan University Hospital from 1997 to 2003
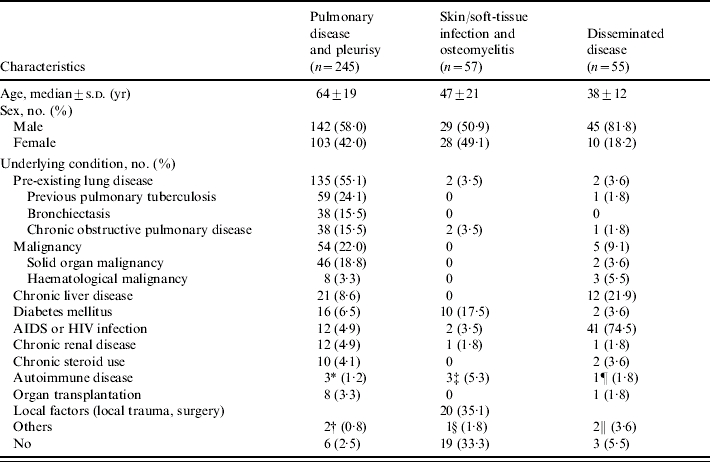
Two systemic lupus erythematous and one rheumatoid arthritis.
One bronchogenic cyst and one pulmonary sequestration.
Two systemic lupus erythematous and one vasculitis.
One mucopolysaccharidosis.
One polymyositis.
One interferon-gamma pathway defect and one monoclonal gammopathy.
MAC was the most common cause of pulmonary disease or pleurisy, followed by RGM (40·4%) and M. kansasii (8·2%) (Table 3). Findings on chest radiographs of these patients were protean including consolidation, interstitial, nodular, and cavitary lesions, and pleural effusion. Isolated pleurisy due to NTM was noted in 18 patients with a predominance of males and a median age of 54 years (range 5–83 years). The most common aetiologies were MAC, followed by M. kansasii and M. fortuitum. Almost half of these patients had underlying malignancy and none of them had AIDS. One patient with pulmonary infection due to M. marinum who presented with a pulmonary mass lesion has been previously reported [10].
Table 3.
Aetiologies associated with five major diseases due to non-tuberculous mycobacteria in National Taiwan University Hospital from 1997 to 2003
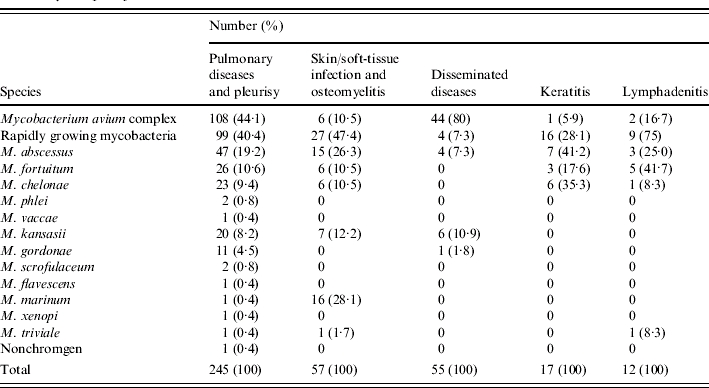
Among the 57 patients with skin/soft-tissue infection and osteomyelitis, 27 were infected with RGM (47·4%), particularly M. abscessus, 16 (28·1%) were infected by M. marinum, and seven (12·2%) were infected with M. kansasii. Thirteen patients (81·3%) had M. marinum infection occurring during 1999–2001 and one each in 1997, 1998, and 2002 respectively. The clinical presentations in these patients included localized abscess at the site of the puncture wounds, skin tumour or papules on the extremities, surgical wound infection, open traumatic wound or fractures. Among these 16 patients with M. marinum infection, 10 (62·5%) had history of exposure to marine environment and animals. Diagnosis was made by culture of the specific pathogen from drainage material or tissue biopsy. MAC accounted for 80% of aetiologies causing disseminated infection, followed by M. kansasii.
Keratitis occurring in 17 patients was caused by RGM in all but one patient (94·1%). Eight (47·1%) of the patients who developed keratitis had received local surgery 3 days to 1 month prior to the diagnosis of keratitis and two patients had previous traumatic injury. Three patients who developed keratitis were contact lens users. Keratitis improved after surgical intervention and local antibiotic treatment in all patients.
Three-quarters of the 12 patients with lymphadenitis were infected with RGM. This disease occurred in younger patients with a median age of 31 years (range 6–64 years). Involved sites included cervical (10 patients), intra-abdominal (one patient), and mediastinal (one patient) regions and the most common presenting symptoms were swelling of lymph nodes, fever, and malaise. Among the 12 patients, five were children (⩽18 years) and all presented with cervical lymphadenitis. One patient had abdominal lymphadenitis caused by M. fortuitum and one had mediastinal lymphadenitis due to M. chelonae.
DISCUSSION
In comparing the findings of the present study regarding NTM diseases during a 7-year period from a university hospital, with those from a previous study regarding NTM diseases in 1992–1996 in the same hospital [7], and with those from other countries [11, 12], six important points were noted.
First, the incidence of NTM diseases increased yearly from 1997 to 2002 in this university hospital. Although the proportion (34·8%) of NTM isolates among all mycobacterial isolates and the percentage (46·2%) of disease-associated NTM isolates among total NTM isolates were high in this study, the proportion of NTM-associated disease among all mycobacterial diseases remained stable (7·5–8·4%, mean 7·7%). This finding was in contrast to the current worldwide trend of an increasing percentage of diseases due to NTM among the total mycobacterial disease [13–15]. This was due to the rising incidence of mycobacterial diseases in the hospital during the study period. The decrease in the incidence of an NTM aetiology of diseases in 2003 might have resulted from the epidemic of severe acute respiratory syndrome (SARS) in Taiwan, which resulted in the widespread implementation of effective infection control measures such as hand hygiene and wearing of masks to prevent spreading of SARS Increased attention to such measures in the community might have reduced the risk of acquisition of NTM. However, it is also possible that patients with NTM infection did not visit the hospital because of the fear of contracting SARS.
Second, the disease spectrum due to NTM differed by geographical regions and with time. Among the diseases caused by NTM, pulmonary disease was the most commonly encountered. The incidence of NTM pulmonary disease was increasing in Taiwan and also in other regions including northern Australia, the United States, and southwest Ireland [5, 12, 15, 16].
Third, the distribution of NTM species associated with clinical diseases also varies. In this study from Taiwan, MAC and RGM were the most commonly encountered pathogens causing diseases. In addition to MAC, which has been reported to be the most common pulmonary pathogen worldwide [13, 15–18], RGM also had similar roles in pulmonary disease in Taiwan. Other studies found that M. kansasii was the second most common NTM causing pulmonary disease in the United States, Japan, Singapore, and some parts of Europe [19–22] and that this organism was the most common pathogen in NTM pulmonary disease in South Africa [23]. Interestingly, M. kansasii was rarely encountered as a pathogen causing pulmonary disease prior to 1997 and this organism ranked third (8·2%) among pathogens causing disease in this study. M. xenopi has recently been noted as one of the most common NTM pulmonary pathogens in parts of England and Canada and has also been reported as a causative pulmonary disease pathogen in parts of the southeastern United States [24]. While M. malmoense has been reported to be the second most common cause of pulmonary disease in northern Europe [25], this organism was not found in this study from Taiwan.
Fourth, RGM were the most important pathogens causing extrapulmonary diseases, particularly skin/soft-tissue infection and osteomyelitis and keratitis. In addition to RGM, this study also demonstrated the emergence of M. marinum and M. kansasii as important pathogens associated with skin/soft-tissue infection and osteomyelitis. These findings are in line with studies from Thailand and France [26, 27].
Fifth, RGM played an important role in lymphadenitis in this study. This finding is different from previous studies, which found that MAC and M. scrofulaceum were the most prevalent NTM pathogens causing lymphadenitis [28, 29]. In this study, M. scrofulaceum did not cause lymphadenitis in any patient.
Finally, AIDS or HIV infection (75%) was the most common risk factor for disseminated disease and 25% of our non-AIDS patients had various underlying immunocompromised conditions. Diabetes mellitus was frequently (17·5%) associated with skin/soft-tissue infection but not with disseminated disease (3·6%) or pulmonary disease (6·5%). Disseminated NTM disease in AIDS patients usually occurred in the advanced immunosuppression stage [30]. The most common pathogens associated with disseminated diseases were MAC and M. kansasii and these diseases were frequently co-existent with pulmonary disease [31, 32]. Four (7·3%) of our patients (only one had AIDS) had disseminated infections due to M. abscessus.
Serositis due to NTM was uncommon in this study. NTM pleurisy has been rarely reported previously [33, 34]. Peritonitis caused by NTM has been reported in patients who underwent continuous ambulatory peritoneal dialysis (CAPD) [35]. In this study, most of our patients (8/12) had underlying malignancy and none of them underwent CAPD. In this study, pleurisy due to NTM was characterized by low virulence and indolence. Only a few of the 10 patients with pleurisy received medical treatment and the mortality was related to underlying conditions. In this study, not all of the 12 patients with NTM-positive culture in ascites received medical treatment. The clinical importance of this situation needs further study.
This retrospective study had two limitations. First, many factors might contribute to the increased incidence of NTM diseases at the hospital from 1997 to 2002, including a real increase in disease burden, a greater awareness of NTM diseases by physicians, and a sensitive and improved laboratory techniques (the introduction of the BACTEC system). The incidence of NTM disease defined in this study used the total number of patients who visited the hospital as the denominator. Obviously, the trend of other related underlying medical conditions of patients, in particular AIDS or HIV infection, use of steroids, organ transplantation, and other immunocompromised conditions, will probably affect the trend of NTM disease. Further studies should be conducted to analyse these confounding factors. Second, definitive diagnosis of pulmonary NTM disease based on the guidelines recommended by the ATS was not feasible in some patients, particularly in those with underlying CLD due to lack of a sufficient number of respiratory specimens for evaluation. Further, the high degree of awareness and cooperation among physicians, surgeons, and microbiologists needed to obtain enough good specimens and to manage these specimens appropriately may have been insufficient in various settings. Further attention to these aspects of management is needed to respond to the emergence of NTM-associated infection.
In conclusion, NTM disease continues to emerge in concert with the wave of a tuberculosis epidemic in Taiwan. This study identified important disease characteristics, changing trends and ranked the clinical importance of the spectrum of NTM isolates and the various diseases they cause in a single university hospital over a recent 7-year period. Further, more effort is needed to monitor the spectrum of diseases caused by NTM in different parts of Taiwan and to find the genetic relationships (molecular typing) of MAC and RGM isolates from humans and those from the environment which serve as reservoirs for these pathogens.
DECLARATION OF INTEREST
None.
REFERENCES
- 1.Lewis AG et al. A clinical study of the chronic lung disease due to nonphotochromogenic acid-fast bacilli. Annals of Internal Medicine. 1960;53:273–285. doi: 10.7326/0003-4819-53-2-273. [DOI] [PubMed] [Google Scholar]
- 2.Wolinsky E. Nontuberculous mycobacteria and associated diseases. American Review of Respiratory Disease. 1979;119:107–159. doi: 10.1164/arrd.1979.119.1.107. [DOI] [PubMed] [Google Scholar]
- 3.Wayne LG, Sramek HA. Agents of newly recognized or infrequently encountered mycobacterial diseases. Clinical Microbiology Reviews. 1992;5:1–25. doi: 10.1128/cmr.5.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.American Thoracic Society. Diagnosis and treatment of disease caused by nontuberculous mycobacteria. American Review of Respiratory Disease. 1990;142:940–953. doi: 10.1164/ajrccm/142.4.940. [DOI] [PubMed] [Google Scholar]
- 5.American Thoracic Society. Diagnosis and treatment of disease caused by nontuberculous mycobacteria. American Journal of Respiratory and Critical Care Medicine. 1997;156:S1–25. doi: 10.1164/ajrccm.156.2.atsstatement. [DOI] [PubMed] [Google Scholar]
- 6.Ottenhoff TH et al. Genetics, cytokines, and human infectious disease: lessons from weakly pathogenic mycobacteria and salmonellae. Nature Gene. 2002;32:97–105. doi: 10.1038/ng0902-97. [DOI] [PubMed] [Google Scholar]
- 7.Shih JY, Hsueh PR, Lee LN et al. Nontuberculous mycobacteria isolates: clinical significance and disease spectrum. Journal of the Formosan Medical Association. 1997;96:621–627. [PubMed] [Google Scholar]
- 8.Vincent V, Murray PR, Baron EJ, Jorgensen JH, Pfaller MA, Yolken RH Mycobacterium: phenotypic and genotypic identification. Manual of Clinical Microbiology. 8th edn. Washington, DC: American Society for Microbiology; 2003. pp. 560–584. : pp. [Google Scholar]
- 9.Turenne CY et al. Necessity of quality-controlled 16S rRNA gene sequence databases: identifying nontuberculous Mycobacterium species. Journal of Clinical Microbiology. 2001;39:3637–3648. doi: 10.1128/JCM.39.10.3637-3648.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lai CC et al. Pulmonary infection due to Mycobacterium marinum in an immunocompetent patient. Clinical Infectious Diseases. 2005;40:206. doi: 10.1086/426693. [DOI] [PubMed] [Google Scholar]
- 11.Henry MT et al. Nontuberculous mycobacteria in non-HIV patients: epidemiology, treatment and response. European Respiratory Journal. 2004;23:741–746. doi: 10.1183/09031936.04.00114004. [DOI] [PubMed] [Google Scholar]
- 12.Kennedy MP et al. Nontuberculous mycobacteria: incidence in Southwest Ireland from 1987 to 2000. Respiratory Medicine. 2003;97:257–263. doi: 10.1053/rmed.2003.1431. [DOI] [PubMed] [Google Scholar]
- 13.Issac-Renton JL et al. Isolation and geographic distribution of Mycobacterium other than Mycobacterium tuberculosis in British Columbia, 1972–1981. Canadian Medical Association Journal. 1985;133:573–576. [PMC free article] [PubMed] [Google Scholar]
- 14.Lamden K et al. Opportunist mycobacteria in England and Wales: 1982 to 1994. Communicable Disease Report. CDR Review. 1996;6:R147–151. [PubMed] [Google Scholar]
- 15.O'Brien DP, Currie BJ, Krause VL. Nontuberculous mycobacterial disease in north Australia: case series and review of the literature. Clinical Infectious Diseases. 2000;31:958–967. doi: 10.1086/318136. [DOI] [PubMed] [Google Scholar]
- 16.O'Brien RJ, Geiter LJ, Snider DE. The epidemiology of nontuberculous mycobacteria diseases in the United States: results from a national survey. American Review of Respiratory Disease. 1987;135:1007–1014. doi: 10.1164/arrd.1987.135.5.1007. [DOI] [PubMed] [Google Scholar]
- 17.Sakatani M. Nontuberculous mycobacteriosis; the present status of epidemiology and clinical studies. Kerkkaku. 1999;74:337–384. [PubMed] [Google Scholar]
- 18.Falkinham JO. Epidemiology of infection by nontuberculous mycobacteria. Clinical Microbiology Review. 1996;9:177–215. doi: 10.1128/cmr.9.2.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tsukamura M et al. Studies on the epidemiology of nontuberculous mycobacteriosis in Japan. American Review of Respiratory Disease. 1988;137:1280–1284. doi: 10.1164/ajrccm/137.6.1280. [DOI] [PubMed] [Google Scholar]
- 20.Mycobacteriosis Research Group of the Japanese National Chest Hospitals. Rapid increase of the incidence of lung disease due to Mycobacterium kansasii in Japan. Chest. 1983;83:890–892. doi: 10.1378/chest.83.6.890. [DOI] [PubMed] [Google Scholar]
- 21.Teo SK, Lo KL. Nontuberculous mycobacterial disease of the lungs in Singapore. Singapore Medical Journal. 1992;33:464–466. [PubMed] [Google Scholar]
- 22.Jarad NA et al. Comparison of characteristics of patients and treatment outcome for pulmonary non-tuberculous mycobacterial infection and pulmonary tuberculosis. Thorax. 1996;51:137–139. doi: 10.1136/thx.51.2.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Corbett EL et al. Nontuberculous mycobacteria: defining disease in a prospective cohort of South African Miners. American Journal of Respiratory and Critical Care Medicine. 1999;160:15–21. doi: 10.1164/ajrccm.160.1.9812080. [DOI] [PubMed] [Google Scholar]
- 24.Faress JA et al. Mycobacterium xenopi pneumonia in the southeastern United States. Southern Medical Journal. 2003;96:596–599. doi: 10.1097/01.SMJ.0000051142.37605.C5. [DOI] [PubMed] [Google Scholar]
- 25.Henriques B et al. Infection with Mycobacterium malmoense in Sweden: report of 221 cases. Clinical Infectious Diseases. 1997;18:596–600. doi: 10.1093/clinids/18.4.596. [DOI] [PubMed] [Google Scholar]
- 26.Zenone T et al. Non-tuberculous mycobacterial tenosynovitis: a review. Scandinavian Journal of Infectious Diseases. 1999;31:221–228. doi: 10.1080/00365549950163482. [DOI] [PubMed] [Google Scholar]
- 27.Mahaisavariya P et al. Nontuberculous mycobacterial skin infections: clinical and bacteriological studies. Journal of the Medical Association of Thailand. 2003;86:52–60. [PubMed] [Google Scholar]
- 28.Haverkamp MH et al. Nontuberculous mycobacterial infection in children: a 2-year prospective surveillance study in the Netherlands. Clinical Infectious Diseases. 2004;39:450–456. doi: 10.1086/422319. [DOI] [PubMed] [Google Scholar]
- 29.Wolinsky E. Mycobacterial lymphadenitis in children: a prospective study of 105 nontuberculous cases with long-term follow-up. Clinical Infectious Diseases. 1995;20:954–963. doi: 10.1093/clinids/20.4.954. [DOI] [PubMed] [Google Scholar]
- 30.Hoover DR et al. An epidemiologic analysis of Mycobacterium avium complex disease in homosexual men infected with human immunodeficiency virus type 1. Clinical Infectious Diseases. 1995;20:1250–1258. doi: 10.1093/clinids/20.5.1250. [DOI] [PubMed] [Google Scholar]
- 31.Horsburgh CJ, Selik RM. The epidemiology of disseminated nontuberculous mycobacterial infection in the Acquired Immunodeficiency Syndrome (AIDS) American Review of Respiratory Disease. 1989;139:4–7. doi: 10.1164/ajrccm/139.1.4. [DOI] [PubMed] [Google Scholar]
- 32.Sherer R et al. Disseminated infection with Mycobacterium kansasii in the Acquired Immunodeficiency Syndrome. Annals of Internal Medicine. 1986;105:710–712. doi: 10.7326/0003-4819-105-5-710. [DOI] [PubMed] [Google Scholar]
- 33.Chau CH, Yew WW, Lam FM. Pleural effusion due to Mycobacterium gordonae infection. International Journal of Tuberculosis and Lung Disease. 2003;7:503. [PubMed] [Google Scholar]
- 34.Gribetz AR et al. Nontuberculous mycobacteria in pleural fluid. Assessment of clinical significance. Chest. 1985;87:495–498. doi: 10.1378/chest.87.4.495. [DOI] [PubMed] [Google Scholar]
- 35.Youmbissi JT et al. Nontuberculous mycobacteria peritonitis in continuous ambulatory peritoneal dialysis. Journal of Nephrology. 2001;14:132–135. [PubMed] [Google Scholar]