SUMMARY
The antibiogram pattern and seasonal distribution of Salmonella serotypes were analysed retrospectively over a 6-year period from January 1999 to December 2004. Blood cultures received in the Bacteriology Laboratory were processed by standard procedures and the Salmonella spp. isolates were identified with specific antisera and standard biochemical tests. Antimicrobial susceptibility testing was carried out by a standard disc diffusion method and the minimum inhibitory concentration (MIC) of ciprofloxacin for 332 representative Salmonella isolates was determined by E test. Salmonella Typhi (75·7%) was the predominant serotype among 830 Salmonella spp. isolated during the study period followed by S. Paratyphi A (23·8%). The maximum number of enteric fever cases occurred during April–June (dry season) followed by July–September (monsoon season). There was a decrease in multidrug-resistant (MDR) S. Typhi, but MDR S. Paratyphi A isolates increased. There was also a dramatic increase in nalidixic acid-resistant isolates. All isolates were susceptible to third-generation cephalosporins and ciprofloxacin except one S. Typhi strain which demonstrated high-level ciprofloxacin resistance with a MIC of 16 μg/ml. A knowledge of the seasonal distribution and antibiotic resistance pattern of Salmonella in a particular geographical region is helpful in the delineation of appropriate control measures required for prevention of enteric fever.
INTRODUCTION
Enteric fever continues to be a global health problem with an estimated 12–33 million cases and 600 000 deaths occurring worldwide each year [1, 2]. In India, it is a major public health problem accounting for more than 300 000 cases per year [3]. Salmonella Typhi is the most common aetiological agent, while S. Paratyphi A is responsible for only a minority of enteric fever cases and is traditionally associated with asymptomatic and/or mild clinical illness [1, 3].
Antibiotic therapy constitutes an integral part of the management of enteric fever since mortality without treatment can be as high as 30%; this can be reduced to <1% by appropriate treatment [4, 5]. Additionally, rates for clinical complications are much higher in multidrug-resistant (MDR) cases (10–40%) [6–8]. Thus, in regions where enteric fever is endemic, it is important to identify those antibiotics to which salmonellae are resistant since resistant strains pose an additional threat by leading to illness of a longer duration [9].
In recent years, some changes in the epidemiology of enteric fever has been noted by various workers. First, there has been a rising incidence of S. Paratyphi A infections [10, 11]. Second, changing trends in antibiotic resistance patterns among salmonellae to conventional first-line antibiotics (ampicillin, co-trimoxazole, chloramphenicol) have been reported from different parts of India [12–15]. The initial report of chloramphenicol resistance and later on of multi-drug resistance in S. Typhi was followed by the re-emergence of chloramphenicol-susceptible strains [12–14]. Subsequently, the emergence of MDR S. Typhi has again been noted with an incidence as high as 60% although there are other reports noting a decline [15]. Another finding of major therapeutic concern is the emergence of reduced ciprofloxacin susceptibility in S. Typhi and S. Paratyphi A and to third-generation cephalosporins such as ceftriaxone which are currently used for the empirical therapy of suspected typhoid fever [16, 17]. Recently, isolated cases of high-level ciprofloxacin resistance in S. Paratyphi A have been reported from South India [18]. Given the variation in the susceptibility pattern reported for S. Typhi and S. Paratyphi A, it is important to monitor these periodically to provide suitable guidelines for treatment.
Enteric fever is endemic in Delhi with a seasonal distribution. Hundreds of laboratory-confirmed cases occur every year peaking during the summer and monsoon seasons [19, 20]. The present study was undertaken to assess the prevalence of Salmonella serotypes in cases of enteric fever, study their antibiotic resistance pattern and note their seasonality.
METHODS
A retrospective study of laboratory records was carried out over a 6-year period from January 1999 to December 2004 at the All India Institute of Medical Sciences, Delhi. This is a 1500-bed tertiary care referral hospital which also caters to a large outpatient population.
Sample collection and processing
Blood samples (1–2 ml from children and 3–5 ml from adults) collected in brain heart infusion broth under aseptic precautions from febrile patients (both outpatients and in-patients) with a clinical diagnosis of enteric fever were received in the Bacteriology Laboratory within 2 h of collection. After overnight incubation at 37°C, subcultures were made on blood agar and MacConkey agar plates at 1, 2 and 10 days to check for growth. Typical lactose non-fermenting colonies were identified as Salmonella by standard biochemical reactions [21] and confirmed by slide agglutination using specific antisera (Murex Diagnostics Ltd, New Delhi, India). Only one isolate per patient was included in the study.
Antimicrobial susceptibility testing
Antimicrobial susceptibility testing of the isolates was carried out by the standard disc diffusion method according to National Committee for Clinical Laboratory Standards (NCCLS) guidelines [22]. The following antibiotics (Himedia Laboratories Ltd, Mumbai, India) were used: ampicillin (10 μg), chloramphenicol (30 μg), co-trimoxazole (1·25/23·75 μg), ceftriaxone (30 μg), nalidixic acid (30 μg) and ciprofloxacin (5 μg); cefixime (5 μg) was added from January, 2003. Escherichia coli ATCC strain 25 922 was used as the quality control strain. Isolates with ‘intermediate’ levels of resistance were included in the percentage of resistant organisms for final analysis. Minimum inhibitory concentration (MIC) of ciprofloxacin was determined by agar dilution method (NCCLS guidelines) and E test strips (AB Biodisk, Solna, Sweden) according to the manufacturer's instructions. A strain was designated as MDR if it exhibited simultaneous resistance to ampicillin, chloramphenicol and co-trimoxazole.
RESULTS
Salmonella isolates
A total of 830 isolates of Salmonella spp. were isolated from 120 211 blood cultures (15 838 in 1999; 16 437 in 2000; 17 958 in 2001; 20 315 in 2002; 24 190 in 2003 and 25 473 in 2004) received during the study period. S. Typhi was the predominant serotype (629, 75·7%) followed by S. Paratyphi A (198, 23·8%). Other Salmonella isolated (3, 0·36%) were S. Enteritidis (2, 0·24%) and S. Typhimurium (1, 0·12%) (Table 1).
Table 1.
Prevalence of Salmonella spp. from January 1999 to December 2004
Demographic characteristics
There were 524 male and 306 female patients (ratio, 1·7:1). The highest number of culture- positive cases was in the 5–19 years age group (377, 45·4%). The number of cases <5 years was 154 (18·5%) and 299 (36·0%) were >19 years of age.
Seasonal occurrence
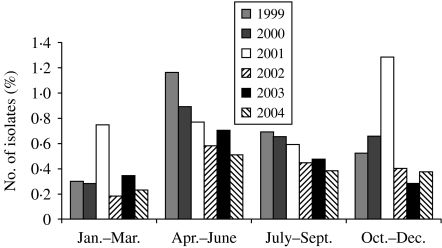
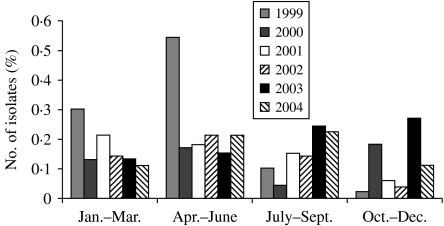
To determine the seasonal occurrence, a year was divided into four equal periods. Incidence was calculated by dividing the number of blood culture-positive cases by the total number of samples received per period. Although enteric fever cases occurred in all months throughout the year, the maximum number occurred during April–June followed by July–September. For typhoid fever, the peak incidence was in April–June in each year except in 2001 when incidence peaked in October–December (Fig. 1). However, for paratyphoid fever, the distribution of cases was not uniform and incidence peaked in different months (Fig. 2).
Fig. 1.
Isolation rates of S. Typhi, by year and month in Delhi, India, 1999–2004.
Fig. 2.
Isolation rates of S. Paratyphi A, by year and month in Delhi, India, 1999–2004.
Resistance to antibiotics
The antimicrobial resistance patterns of S. Typhi and S. Paratyphi A are shown in Tables 2 and 3 respectively. In case of S. Typhi, ampicillin and nalidixic acid resistance showed an upward trend over the years. There was an initial increase in co-trimoxazole and chloramphenicol resistance until 2001 but this decreased in the years following (Table 2). There was also a decrease in MDR S. Typhi which may have been linked to a decrease in the number of chloramphenicol-resistant strains. In contrast, resistance of S. Paratyphi A to all the first-generation drugs increased consistently over the years with a simultaneous increase in MDR isolates (Table 3). There was also a dramatic rise in nalidixic acid-resistant (NAR) isolates. For S. Typhi, 171 out of 466 (36·7%) NAR isolates were MDR and for S. Paratyphi A, 10 out of 154 (6·5%) NAR isolates were MDR. All S. Typhi and S. Paratyphi A isolates were susceptible to the cephalosporins.
Table 2.
Resistance (%) of S. Typhi to various antibiotics (1999–2004)
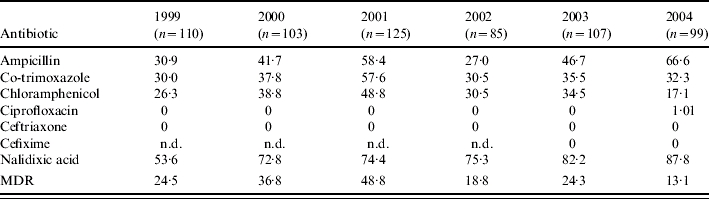
MDR, multidrug resistant; n.d., not done.
Table 3.
Resistance (%) of S. Paratyphi A to various antibiotics (1999–2004)
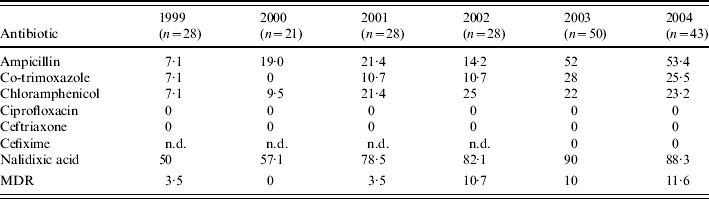
MDR, multidrug resistant; n.d., not done.
Regarding ciprofloxacin, all isolates of Salmonella except one of S. Typhi were susceptible to ciprofloxacin by disc diffusion and agar dilution (diameter⩾21 mm, MIC⩽1·0 μg/ml) according to NCCLS guidelines [22]. Of the ciprofloxacin-sensitive isolates, a representative 332 isolates were also tested by E test and all were found to be susceptible. However, one S. Typhi strain isolated in 2004 demonstrated high-level ciprofloxacin resistance with a MIC value of 16 μg/ml by E test [23].
A comparison of the ciprofloxacin MIC values for the representative NAR and nalidixic acid- sensitive (NAS) strains showed that the MICs of NAR strains was about tenfold higher than for NAS strains although there was some overlap between the two groups (Table 4). Furthermore, the MIC of ciprofloxacin for S. Paratyphi A strains (median 0·25 μg/ml, absolute range 0·023–1·0 μg/ml, interquartile range 0·19–0·38 μg/ml) was significantly higher than that of the S. Typhi strains (median 0·125 μg/ml, absolute range 0·002–1·0 μg/ml, interquartile range 0·023–0·19 μg/ml).
Table 4.
MIC of ciprofloxacin for nalidixic acid susceptible and resistant isolates
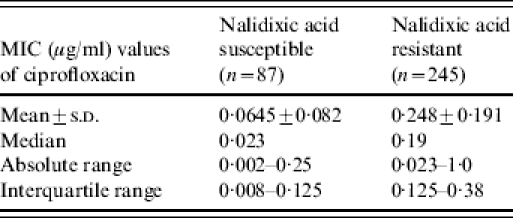
MIC, Minimum inhibitory concentration.
DISCUSSION
In the current study, S. Typhi and S. Paratyphi A were responsible for a total of 75·7% and 23·8% of cases of enteric fever respectively. However, the proportion of S. Paratyphi A isolates rose from 20·3% in 1999 to 30·3% in 2004. This is in contrast to a recently published study [24] from North India where the proportion of S. Paratyphi A isolated from enteric fever cases remained almost constant throughout the study period from 1997 to 2001. However, our findings are in accordance with a study from South India [25] where the incidence of S. Paratyphi A increased steadily from 1998 to 2001. Progressive replacement of bivalent TA vaccine (with protective immunity against both S. Typhi and S. Paratyphi A) with monovalent vaccines effective only against S. Typhi is probably responsible for the increase in prevalence noted for S. Paratyphi A [25]. In addition, the increasing use of other S. Typhi vaccines, namely, the oral Ty21a and the Vi polysaccharide vaccine in the general population has presumably led to a decline in enteric fever cases due to S. Typhi.
The highest incidence of enteric fever occurs in the 5–19 years age group. After age 20, the incidence falls, due probably to acquisition of immunity from clinical or subclinical infection [26]. In the current study, although the highest number of culture-positive cases occurred in persons aged 5–19 years, more than half of the cases combined were <5 and >19 years of age. These findings suggest a need to review the current strategies for vaccination against enteric fever which needs to be administered in a manner that confers protection to both the age ranges. However, all three licensed typhoid vaccines (whole-cell heat phenol-inactivated vaccine, Ty21a, and the Vi vaccine) have limitations which restrict their use for routine childhood immunization <5 years [27].
We noted the maximum incidence of typhoid fever in the summer months (April–June) followed by the monsoon season (July–September). Recent studies from India [24] and elsewhere [27–29] have also noted increased isolation particularly at the end of the summer months. During the dry season, the water level gets progressively lower, becomes more stagnant and potable quality deteriorates as the weather becomes hotter [27]. Under these conditions the likelihood of ingesting Salmonella from contaminated water is high. The non-uniformity in distribution of S. Paratyphi A cases is in accordance to that noted earlier [20].
Regarding antimicrobial susceptibility, in the case of S. Typhi there appears to be a re-emergence of susceptibility to co-trimoxazole and chloramphenicol, thus leading to a decrease in MDR strains from 24·5% in 1999 to 13·1% in 2004. A decrease in occurrence of MDR S. Typhi has also been noted in South India [15]. However, the consistent increase in resistance year on year to all three first-line drugs for S. Paratyphi A with a concomitant increase in MDR strains is a matter of concern. Another feature of importance is the marked increase in NAR strains in both S. Typhi and S. Paratyphi A. Nalidixic acid susceptibility has been validated as a screening test for reduced susceptibility to ciprofloxacin and as observed here, nalidixic acid resistance is associated with a high MIC of ciprofloxacin which in turn is associated with treatment failure [30, 31]. The finding of high-level ciprofloxacin resistance in S. Typhi [23] underlines the need to recognize the possible emergence of more such strains in the near future. Although all Salmonella strains in the current study were fully susceptible to both the extended spectrum cephalosporins tested, attention should also be paid to the emergence of strains resistant to these antibiotics in the near future.
It is amply clear from the present study that long-term surveillance programmes are essential to identify changes in the epidemiology of enteric fever and to monitor trends in antimicrobial resistance patterns of Salmonella strains isolated in endemic localities. The gradual increase in the incidence of S. Paratyphi A, the emergence of S. Typhi strains fully resistant to ciprofloxacin and the increase of multidrug resistance in S. Paratyphi A are clear indications that this disease is far from conquered. In particular, S. Paratyphi A no longer appears to be a mild disease. Thus, a knowledge of seasonal distribution and antibiotic resistance pattern of Salmonella isolates in particular geographical regions is most helpful in the delineation of appropriate control measures required for prevention of enteric fever.
DECLARATION OF INTEREST
None.
References
- 1.Miller SI, Pegues DA, Mandell GL, Bennet JE, Dolin R. Principles and Practice of Infectious Disease. 4th edn. New York: Churchill Livingstone; 1998. Salmonella species, including Salmonella typhi; pp. 2344–2373. : pp. [Google Scholar]
- 2.Ivanoff B. Typhoid fever, global situation and WHO recommendations. Southeast Asian Journal of Tropical Medicine and Public Health. 1995;26:1–6. (Suppl 2): [Google Scholar]
- 3.Richens J, Weatherale DJ, Ledingham JGG, Warrell DA. Oxford Textbook of Medicine. 3rd edn. Vol. 1. London: Oxford Medical Publication; 1996. Typhoid and paratyphoid fevers; pp. 560–568. , vol. : pp. [Google Scholar]
- 4.Mirza SH. The prevalence and clinical features of multi-drug resistant Salmonella typhi infections in Baluchistan, Pakistan. Annals of Tropical Medicine and Parasitology. 1995;89:515–519. doi: 10.1080/00034983.1995.11812984. [DOI] [PubMed] [Google Scholar]
- 5.Mermin JH et al. Typhoid fever in the United States 1985–1994: changing risks of international travel and increasing antimicrobial resistance. Archives of Internal Medicine. 1998;158:633–638. doi: 10.1001/archinte.158.6.633. [DOI] [PubMed] [Google Scholar]
- 6.Kabra SK, Talati A, Patel S. Multidrug-resistant typhoid fever. Tropical Doctor. 2000;30:195–197. doi: 10.1177/004947550003000404. [DOI] [PubMed] [Google Scholar]
- 7.El Sherbini A. An outbreak of typhoid fever resistant to chloramphenicol and other drugs in Egypt. Journal of Tropical Pediatrics. 1992;38:331–334. doi: 10.1093/tropej/38.6.331. [DOI] [PubMed] [Google Scholar]
- 8.Koul PB et al. Multi drug resistant Salmonella Typhi infection: clinical profile and therapy. Indian Pediatrics. 1991;28:357–361. [PubMed] [Google Scholar]
- 9.Khosla SN et al. Drug resistant typhoid fever. Tropical Doctor. 1998;28:235–237. doi: 10.1177/004947559802800419. [DOI] [PubMed] [Google Scholar]
- 10.Sood S et al. Paratyphoid fever in India: an emerging problem. Emerging Infectious Diseases. 1999;5:483–484. doi: 10.3201/eid0503.990329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tankhiwale SS, Agrawal G, Jalgaonkar SV. An unusually high occurrence of Salmonella enterica serotype Paratyphi A in patients with enteric fever. Indian Journal of Medical Research. 2003;117:10–12. [PubMed] [Google Scholar]
- 12.Agarwal SC. Chloramphenciol resistance of Salmonella species in India, 1956–61. Bulletin of the World Health Organization. 1962;17:331–335. [PMC free article] [PubMed] [Google Scholar]
- 13.Panicker CKJ, Vimla KN. Transferable chloramphenicol resistance in Salmonella Typhi. Nature. 1972;239:109–110. doi: 10.1038/239109b0. [DOI] [PubMed] [Google Scholar]
- 14.Sood S et al. Re-emergence of chloramphenicol sensitive Salmonella typhi. Lancet. 1999;353:1241–1242. doi: 10.1016/s0140-6736(99)00637-6. [DOI] [PubMed] [Google Scholar]
- 15.Madhulika U, Harish BN, Parija SC. Current pattern in antimicrobial susceptibility of Salmonella Typhi isolates in Pondicherry. Indian Journal of Medical Research. 2004;120:111–114. [PubMed] [Google Scholar]
- 16.Renuka K et al. Reduced susceptibility to ciprofloxacin and gyr A gene mutation in North Indian strains of Salmonella enterica serotype Typhi and serotype Paratyphi A. Microbial Drug Resistance. 2004;10:146–153. doi: 10.1089/1076629041310028. [DOI] [PubMed] [Google Scholar]
- 17.Saha SK et al. A highly ceftriaxone-resistant Salmonella Typhi in Bangladesh. Pediatric Infectious Disease Journal. 1999;18:387. doi: 10.1097/00006454-199904000-00018. [DOI] [PubMed] [Google Scholar]
- 18.Harish BN, Madhulika U, Parija SC. Isolated high-level ciprofloxacin resistance in Salmonella enterica subsp. enterica serotype Paratyphi A. Journal of Medical Microbiology. 2004;53:819. doi: 10.1099/jmm.0.05451-0. [DOI] [PubMed] [Google Scholar]
- 19.Sinha A et al. Typhoid fever in children aged less than 5 years. Lancet. 1999;354:734–737. doi: 10.1016/S0140-6736(98)09001-1. [DOI] [PubMed] [Google Scholar]
- 20.Singh B, Sharma MD, Saxena SN. Paratyphoid A fever in Delhi: monthly incidence. Indian Journal of Medical Research. 1965;53:938–941. [PubMed] [Google Scholar]
- 21.Old DC, Collee JG, Fraser AG, Marmion BP, Simmons A. Mackie and McCartney Practical Medical Microbiology. 14th edn. London: Churchill Livingstone; 1996. Salmonella; pp. 385–404. : pp. [Google Scholar]
- 22.National Committee for Clinical Laboratory Standards (NCCLS) Villanova, PA: 2004. . Performance standards for antimicrobial disk susceptibility testing. 14th Informational Supplement (M100-S14). [Google Scholar]
- 23.Renuka K et al. High-level ciprofloxacin resistance in Salmonella enterica serotype Typhi in India. Journal of Medical Microbiology. 2005;54:999–1000. doi: 10.1099/jmm.0.45966-0. [DOI] [PubMed] [Google Scholar]
- 24.Gautam V et al. Sensitivity pattern of Salmonella serotypes in Northern India. Brazilian Journal of Infectious Diseases. 2002;6:281–287. doi: 10.1590/s1413-86702002000600003. [DOI] [PubMed] [Google Scholar]
- 25.Padmapriya V, Kenneth J, Amarnath SK. Re-emergence of Salmonella Paratyphi A: a shift in immunity. National Medical Journal of India. 2003;16:47–48. [PubMed] [Google Scholar]
- 26.Park K. Park's Textbook of Preventive and Social Medicine. 18th edn. Jabalpur: Bhanot; 2005. Typhoid fever; pp. 187–190. : pp. [Google Scholar]
- 27.Lin FYC et al. The epidemiology of typhoid fever in the Dong Thap Province, Mekong Delta region of Vietnam. American Journal of Tropical Medicine and Hygiene. 2000;62:644–648. doi: 10.4269/ajtmh.2000.62.644. [DOI] [PubMed] [Google Scholar]
- 28.Ollé-Goig JE, Ruiz L. Typhoid fever in rural Haiti. Bulletin of the Pan American Health Organization. 1993;27:382–388. [PubMed] [Google Scholar]
- 29.Velema JP et al. Typhoid fever in Ujung Pandang, Indonesia – high-risk groups and high-risk behaviours. Tropical Medicine and International Health. 1997;2:1088–1094. doi: 10.1046/j.1365-3156.1997.d01-179.x. [DOI] [PubMed] [Google Scholar]
- 30.Hakanen A et al. Detection of decreased fluoroquinolone susceptibility in Salmonellas and validation of nalidixic acid screening test. Journal of Clinical Microbiology. 1999;37:3572–3577. doi: 10.1128/jcm.37.11.3572-3577.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kapil A, Renuka D, Das B. Nalidixic acid susceptibility test to screen ciprofloxacin resistance in Salmonella Typhi. Indian Journal of Medical Research. 2002;115:49–54. [PubMed] [Google Scholar]