SUMMARY
In this study, we used plasmid profile analysis, XbaI macrorestriction with pulsed-field gel electrophoresis (PFGE), and PCR of the ipaH gene, to study the molecular characteristics of 183 Shigella spp. isolated during May 2000 to April 2003 from rectal swabs of patients with watery and/or bloody diarrhoea in a new industrialized area of Thailand. Among the 183 isolates, 167 were S. sonnei and 16 were S. flexneri. For plasmid profile analysis, the 183 isolates revealed 16 different plasmid patterns, designated patterns A to P. The sizes of the plasmid bands were: 6, 5·5, 5, 4·5, 4, 3·25, 2·75, 2·5, 2, 1·75, 1·5 and/or 1·25 kb. The frequency of each plasmid band was 4·5 kb (165 isolates), 3·25 kb (161 isolates), 5·5 kb (129 isolates), 1·75 kb (121 isolates), 1·5 kb (35 isolates), 5 kb (21 isolates), 2 kb (16 isolates), 2·75 kb (12 isolates), 1·25 kb (9 isolates), and 6 kb (8 isolates). PFGE analysis revealed 45 different XbaI macrorestricted DNA banding patterns which could be grouped into 11 groups. All the isolates gave PCR amplicons of the ipaH gene. Plasmid profile analysis and PFGE are powerful tools for differentiation of the Shigella spp. This study provides important data on the molecular characteristics of Shigella isolates in Thailand, which could be useful as an epidemiological baseline for identifying relationships with strains that may emerge in the future.
INTRODUCTION
It is known that mildest form of shigellosis is caused by strains of Shigella boydii and S. sonnei, which are predominant in the more developed, industrialized areas of the world [1–3]. S. flexneri strains are responsible for endemic shigellosis in less developed regions [4]. In developing countries, epidemic shigellosis is almost always caused by Shiga toxin-producing S. dysenteriae type 1 (Sd1). These epidemics can be extremely large and prolonged, often with a high fatality rate, especially in young children, the elderly, and immunocompromised individuals, with devastating public health consequences [5].
For the first time in 20 years, Thailand experienced Sd1 epidemics in the northeastern provinces in the autumn of 1986 and again in the spring and autumn of 1987. The epidemic strain was resistant to tetracycline, streptomycin, chloramphenicol, and trimethoprim–sulphamethoxazole. There were two deaths among the 101 hospitalized, culture-confirmed cases [6]. In July 1992, there was an emergence of nalidixic acid-resistant Sd1 in a western district of Thailand near the Myanmar border, when 485 schoolchildren contracted dysentery. Coconut milk dessert prepared at a school was identified as the vehicle of transmission [7]. For endemic shigellosis, S. flexneri was the main aetiological agent in the past; however, since the last decade of the twentieth century, S. boydii and S. sonnei have been responsible for more shigellosis in the country [8]. The incidence of bacillary dysentery officially recorded by the Department of Epidemiology, Ministry of Public Health, Thailand, based on isolation of Shigella from stool samples containing blood and mucus, was ∼2000 cases annually, a case rate of 3·345/100 000 population [8]. These are under-reported data, as it is generally recognized that the clinical features of shigellosis may also include watery stool without blood or mucus, especially those caused by S. boydii and S. sonnei. In this study, serological and molecular characterization of Shigella isolates from patients with watery and/or bloody diarrhoea was done. The patients lived in a new industrialized area of Thailand, the Kaeng-Khoi district of Saraburi province, located ∼100 km northeast of Bangkok. Methods included plasmid profile analysis [9], XbaI macrorestriction with pulsed-field gel electrophoresis (PFGE) [10], and DNA amplification of the ipaH gene by polymerase chain reaction (PCR) [11].
MATERIALS AND METHODS
Study area and bacterial strains
Shigellosis in the Kaeng-Khoi district of Saraburi province was studied from May 2000 to April 2003. The area is a small city surrounded by rural villages that depend primarily on agriculture for income; in addition, there are also large industrial areas. The Thailand government census in 2003 indicated that there was a total of population of 80 141 in this area, including 39 594 males and 40 547 females. There were 5686 children aged <5 years. The area has 20 community health centres and two hospitals.
A total of 183 Shigella isolates were obtained. They were initially characterized according to serogroups, serotypes and/or phases (for S. sonnei strains) using specific sera (Denka Seiken, Tokyo, Japan). The bacteria were then characterized by plasmid profile analysis, PFGE and DNA amplification of the ipaH gene. Shigella strain SH145 was used as a positive control in the PCR for amplification of the ipaH gene [12]. This strain was from the Armed Forces Research Institute of Medical Science (AFRIMS), Bangkok, Thailand.
Plasmid profile analysis
Analysis of plasmids was performed as previously described [9]. Plasmid DNA from each isolate was prepared by rapid alkaline lysis procedure [13]. The method relies on alkaline SDS to free the plasmid DNA from the cell. Each plasmid preparation was fractionated in 0·5% agarose gel (Seakem¯ LE, Rockland, ME, USA) in TAE buffer and the gel was stained with 0·5 μg/ml ethidium bromide. The plasmid profiles were visualized using Gel Doc 2000 (Bio-Rad Laboratories, Richmond, CA, USA). One-kb DNA marker (Fermentas, Ontario, Canada) was used as sized standards. Because large plasmids of Shigella spp. tend to be lost during storage and subculturing or plasmid extraction, only plasmid bands below the chromosomal DNA band were taken into account for plasmid profile analysis [14].
PFGE
Genomic DNA for PFGE was prepared in agarose plugs using the method previously described [10]. Slices of agarose plug were digested with 20 U XbaI restriction endonuclease (Fermentas). The restriction fragments were separated by using the contour-clamped homogeneous electric field method on a CHEF MAPPER System (Bio-Rad) under predetermined conditions (21 h at a temperature of 14°C, 120° constant angle, 6 V/cm and with ramped pulsed time of 2–30 s). After electrophoresis, the gel was stained with 0·5 mg/ml ethidium bromide (Sigma-Aldrich, St. Louis, MO, USA) in 0·5×TBE for 20 min, destained in 0·5×TBE for 30 min, viewed and photographed under Gel Doc 2000 (Bio-Rad). Concatemers of the λ phage DNA ladder (Promega, Madison, WI, USA) starting at 50 kb was used as a molecular size marker. The chromosomal restriction PFGE profiles were classified visually into banding patterns on the basis of differences in numbers and locations of the DNA fragments.
PCR
PCR was used for detecting ipaH in the 183 Shigella isolates. The target gene is unique to members of the genus, and is also a marker for invasiveness into host intestinal cells; the gene is present in multiple copies in both plasmids and chromosomes [15]. The primer sequences for amplification of this gene were, forward: GTT CCT TGA CCG CCT TTC CGA TA, and reverse: GCC GGT CAG CCA CCC TA [12]. The PCR mixture contained 2·5 μl 10×buffer (1·5 mm MgCl2, 0·2 mm dNTPs (dATP, dGTP, dCTP, and dTTP) (Sib Enzyme Ltd, Novosibirk, Russia), 1 μ m of individual primers (Amersham Biosciences, Piscataway, NJ, USA), 0·75 U Taq polymerase (Finnzymes Oy, Espoo, Finland) and 3 μl of DNA templates. The final volume was adjusted by adding sterilized distilled water to 25 μl. The mixture was processed in a thermocycler (Eppendorf Mastercycler Gradient, Foster City, CA, USA) for DNA amplification. The steps and condition of the PCR assay for DNA amplification were: denaturation at 94°C for 60 s, annealing at 60°C for 90 s, extension at 72°C for 60 s and final extension at 72°C for 10 min [9]. The expected size of the PCR amplicon was 500 bp and was revealed by Gel Doc 2000 (Bio-Rad). The size of the DNA amplicon was determined by comparison with the standard marker [11, 12].
RESULTS
Serogroups and serotypes of the Shigella spp.
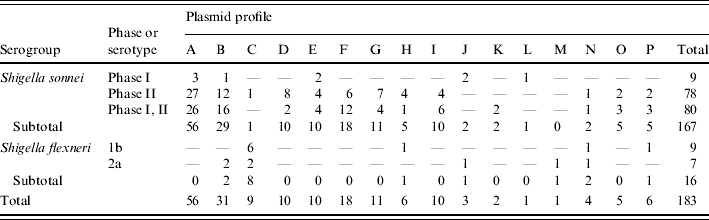
Of the 183 isolates, 167 were S. sonnei (nine strains were phase I, 78 strains were phase II, and 80 strains were phase I, II) and 16 isolates were S. flexneri (nine strains were serotype 1b, and seven strains were serotype 2a).
Plasmid profile analysis
The results of plasmid analysis revealed that the 183 isolates had 16 different plasmid profile patterns, designated patterns A to P (Table 1). Fifty-six S. sonnei strains had pattern A (four plasmid bands: 5·5, 4·5, 3·25, and 1·75 kb). Thirty-one strains had plasmid profile pattern B, which consisted of three plasmid bands (5·5, 4·5 and 3·25 kb), and 18 strains had plasmid profile patterns F, which were five plasmid bands (5·5, 4·5, 3·25, 1·75 and 1·5 kb). The rest of the strains had plasmid profile patterns G (11 strains), E (10 strains), I (10 strains), D (10 strains), C (9 strains), H (6 strains), P (6 strains), J (3 strains), O (5 strains), N (4 strains), K (2 strains), L (1 strains), and M (1 strain) (Table 2).
Table 1.
Sizes of plasmids in each of the 16 plasmid profiles of the 183 Shigella isolates
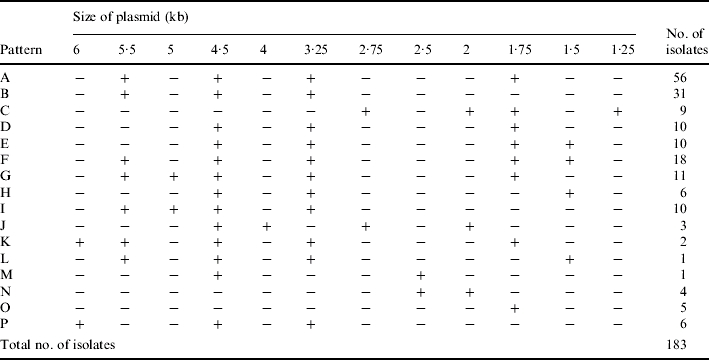
Table 2.
Serogroups, serotypes/phases, and plasmid profiles of the 183 Shigella isolates
PFGE
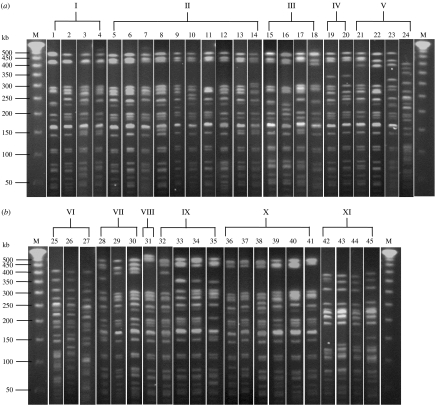
The 183 isolates had 45 different PFGE banding patterns which can be grouped into 11 groups (Fig. 1, Table 3). Fifty S. sonnei isolates had DNA banding pattern 6, while 28, 24, 12, 6, and 4 of the remaining S. sonnei isolates had patterns 13, 5, 9, 19, and 22 respectively. One isolate each of seven S. flexneri 2a showed patterns 10, 13, 25, 34, 35, 37 and 42. Three S. flexneri 1b isolates had pattern 42, two had pattern 19 and one each of the remaining 4 S. flexneri 1b isolates had patterns 18, 25, 36 and 43. Table 4 summarizes the serogroups, serotypes, plasmid profiles, and PFGE profiles of all isolates.
Fig. 1.
The 45 pulsed-field gel electrophoresis (PFGE) banding patterns of the 183 Shigella isolates, which were grouped into 11 PFGE groups. (a) Lane M, molecular weight marker (lambda phage DNA ladder), lanes 1–23; PFGE banding patterns 1–24 respectively. (b) Lane M, molecular weight marker, lanes 25–45, PFGE banding patterns 25–45 respectively. The PFGE banding patterns 1–4, 5–14, 15–18, 19–20, 21–24, 25–27, 28–30, 31, 32–35, 36–41, and 42–45 were grouped into 11 groups, i.e. PFGE groups I–XI respectively [(a) and (b)].
Table 3.
Pulsed-field gel electrophoresis (PFGE) groups of the 183 Shigella isolates
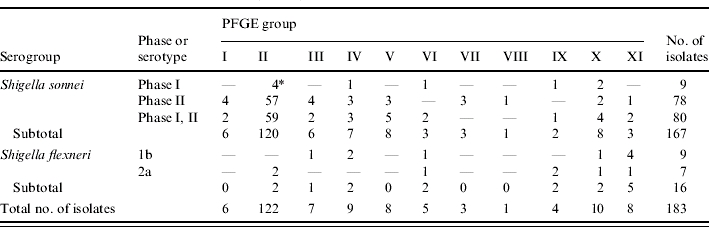
No. of isolates with the indicated PFGE group.
Table 4.
Comparison of serogroups, serotypes/phases, plasmid profiles, and pulsed-field gel electrophoresis (PFGE) groups of the 183 Shigella isolates

PCR
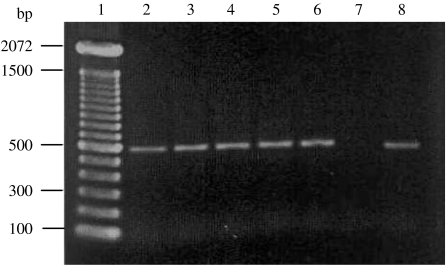
All 183 isolates produced PCR amplicons of the ipaH gene of 500 bp (Fig. 2).
Fig. 2.
The PCR amplicon of IpaH at ∼500 bp. Lane 1, DNA marker; lanes 2 and 3, Shigella flexneri 2a and 1b respectively; lanes 4–6, Shigella sonnei phases I, II and I, II respectively; lane 7, negative control; lane 8, positive control (SH145 strain).
DISCUSSION
In this study, Shigella strains were isolated from rectal swab samples of patients with watery and/or bloody diarrhoea. The patients were inhabitants of Kaeng-Khoi district, which is a new industrialized town in lower northeastern Thailand. The majority of the isolates were found to be S. sonnei (91·25%), while the remainder (8·75%) were S. flexneri. The finding conforms with the previous investigation of shigellosis in a coastline province of Thailand where cases of shigellosis caused by S. sonnei and S. boydii outnumbered the cases caused by S. flexneri [16], and agreed well with the data for the whole country [8].
Shigellae usually harbour various plasmids, such as those required for bacterial invasion into the host intestinal epithelial cells and antibiotic resistance [17]. As many as 10 plasmids were contained in one strain [18]. Thus, plasmid profile analysis is one of the appropriate methods for molecular characterization of shigellae, while the technique is less useful for the bacteria that have only a few or no plasmids, e.g. Vibrio cholerae. However, it must be noted that plasmids are highly mobile genetic elements and bacteria may be cured, or they may acquire additional plasmids at any time. Thus, differentiation of the strains should not rely on plasmid profiling, and an additional molecular method should be used. In this study, plasmid profile analysis and PFGE were used to characterize Shigella isolates from patients with diarrhoea. With the plasmid analysis, up to different 16 profiles were identified amongst the 183 isolates. Results conformed with the data reported elsewhere that Shigella spp. harbours heterogeneous population of plasmids [17, 18].
Previous studies have shown that PFGE has high discriminatory power in the molecular characterization of several enteric bacteria including Shigella. The method has been used as a reference epidemiological tool in conjunction with other methods, e.g. plasmid profile analysis, and antibiotic susceptibility biogram for differentiation of Shigella spp. [17, 19]. Restriction endonucleases regularly used include NotI and XbaI [14, 17, 20]. In this study, the XbaI-digested genomic DNA fragments of the 183 isolates were analysed using PFGE. As many as 45 different DNA banding patterns, which could be arbitrarily grouped into 11 groups, were revealed. PFGE seemed to have higher discriminatory power than the plasmid profile analysis, however, there was no correlation between the PFGE patterns and plasmid profiles (Table 4). All isolates were confirmed by PCR to be pathogenic by the presence of the ipaH gene [11, 15, 21, 22].
Shigella in Thailand has never been characterized by PFGE and plasmid profile analysis. Thus, our data provides baseline molecular markers which can be useful in the shigellosis surveillance.
ACKNOWLEDGEMENTS
The work was financially co-supported by the Thailand Research Fund (TRF), Thailand, and the International Vaccine Institute, Seoul, Korea. The authors thank Dr Orntipa Sethabutr of AFRIMS, Bangkok, for providing the SH145 Shigella strain.
DECLARATION OF INTEREST
None.
REFERENCES
- 1.Preston MA, Borczyk AA. Genetic variability and molecular typing of Shigella sonnei strains isolated in Canada. Journal of Clinical Microbiology. 1994;32:1427–1430. doi: 10.1128/jcm.32.6.1427-1430.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Centers for Disease Control and Prevention (CDC) Shigella surveillance: annual tabulation summary, 1999. Atlanta, Georgia: US Department of Health and Human Services, CDC; 2000. [Google Scholar]
- 3.Lee LA et al. Hyperendemic shigellosis in United States: a review of surveillance data for 1967–1988. Journal of Infectious Diseases. 1991;64:894–900. doi: 10.1093/infdis/164.5.894. [DOI] [PubMed] [Google Scholar]
- 4.Kotloff KL et al. Global burden of Shigella infections: implications for vaccine development and implementation of control strategies. Bulletin of the World Health Organizaton. 1999;77:651–666. [PMC free article] [PubMed] [Google Scholar]
- 5.Bennish ML, Wojtyniak BJ. Mortality due to shigellosis: community and hospital data. Review of Infectious Diseases. 1991;4:245–251. doi: 10.1093/clinids/13.supplement_4.s245. [DOI] [PubMed] [Google Scholar]
- 6.Taylor DN et al. Introduction and spread of multi-resistant Shigella dysenteriae I in Thailand. American Journal of Tropical Medicine and Hygiene. 1989;40:77–85. doi: 10.4269/ajtmh.1989.40.77. [DOI] [PubMed] [Google Scholar]
- 7.Hoge CW et al. Emergence of nalidixic acid resistant Shigella dysenteriae type 1 in Thailand: an outbreak associated with consumption of a coconut milk dessert. International Journal of Epidemiology. 1995;24:1228–1232. doi: 10.1093/ije/24.6.1228. [DOI] [PubMed] [Google Scholar]
- 8.Ministry of Public Health of Thailand 2002. . Annual Report,
- 9.Panutdaporn N et al. Genotypes and phenotypes of shiga toxin-producing Escherichia coli isolated from healthy cattle in Thailand. Journal of Infection. 2004;48:149–160. doi: 10.1016/j.jinf.2003.08.011. [DOI] [PubMed] [Google Scholar]
- 10.Koonpaew S et al. Genome fingerprinting by pulsed-field gel electrophoresis of isolates of Burkholderia pseudomallei from patients with melioidosis in Thailand. Acta Tropica. 2000;74:187–191. doi: 10.1016/s0001-706x(99)00069-8. [DOI] [PubMed] [Google Scholar]
- 11.Islam MS et al. Detection of shigellae from stools of dysentery patients by culture and polymerase chain reaction techniques. Journal of Diarrhoeal Diseases Research. 1998;16:248–251. [PubMed] [Google Scholar]
- 12.Sethabutr O et al. Detection of Shigella and enteroinvasive Escherichia coli by PCR in the stools of patients with dysentery in Thailand. Journal of Diarrhoeal Diseases Research. 1994;12:265–269. [PubMed] [Google Scholar]
- 13.Birnboim HC. A rapid alkaline extraction method for the isolation of plasmid DNA. Methods in Enzymology. 1983;100:243–255. doi: 10.1016/0076-6879(83)00059-2. [DOI] [PubMed] [Google Scholar]
- 14.Chiou C et al. Molecular epidemiology of a Shigella flexneri outbreak in a mountainous township in Taiwan, Republic of China. Journal of Clinical Microbiology. 2001;39:1048–1056. doi: 10.1128/JCM.39.3.1048-1056.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Venkatesan MM et al. Use of Shigella flexneri ipaC and ipaH gene sequences for the general identification of Shigella spp. and enteroinvasive Escherichia coli. Journal of Clinical Microbiology. 1989;27:2687–2691. doi: 10.1128/jcm.27.12.2687-2691.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sonjai K et al. Validation of salmonellosis and shigellosis diagnostic test kits at a provincial hospital in Thailand. Asian Pacific Journal of Allergy and Immunology. 2001;19:115–127. [PubMed] [Google Scholar]
- 17.Navia MM et al. Typing and characterization of mechanisms of resistance of Shigella spp. isolated from feces of children under 5 years of age from Ifakara, Tanzania. Journal of Clinical Microbiology. 1999;37:3113–3117. doi: 10.1128/jcm.37.10.3113-3117.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jamieson AF et al. Characterization of plasmids from antibiotic resistant Shigella isolates by agarose gel electrophoresis. Journal of General Microbiology. 1979;113:73–81. doi: 10.1099/00221287-113-1-73. [DOI] [PubMed] [Google Scholar]
- 19.Brian MJ et al. Evaluation of the molecular epidemiology of an outbreak of multiply resistant Shigella sonnei in a day-care center by using pulsed-field gel electrophoresis and plasmid DNA analysis. Journal of Clinical Microbiology. 1993;31:2152–2156. doi: 10.1128/jcm.31.8.2152-2156.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Houang ETS et al. Study of the relatedness of isolates of Shigella flexneri and Shigella sonnei obtained in 1986 and 1987 and in 1994 and 1995 from Hong Kong. Journal of Clinical Microbiology. 1998;36:2404–2407. doi: 10.1128/jcm.36.9.2404-2407.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Oyofo BA et al. Detection of enterotoxigenic Escherichia coli, Shigella and Campylobacter spp. by multiplex PCR assay. Journal of Diarrhoeal Diseases Research. 1996;14:207–210. [PubMed] [Google Scholar]
- 22.Thong KL et al. Detection of virulence genes in Malaysian Shigella species by multiplex PCR assay. http://www.biomedcentral.com/1471-2334/5/8. Bio-Medical Central Infectious Diseases. 2005;5:8. doi: 10.1186/1471-2334-5-8. ). Accessed 12 November 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]