SUMMARY
In 2001, two residents of a nursing home in Lower Saxony, Germany, were diagnosed with acute hepatitis B virus (HBV) infection. A systematic contact investigation of 188 residents yielded 19 confirmed or probable cases of acute or recent HBV infection and three persistent asymptomatic HBsAg carriers. Sequence analysis revealed that one carrier had high viraemia (109 genomes/ml), HBV genotype A2, and the same S gene and/or X gene sequence as 16 acutely infected persons. An unmatched case-control study was conducted with the 17 cases that had sequence identity together with 26 controls. The strongest association was found for treatment by a particular general practitioner (GP) (OR>11, P<0·001) and blood sampling for glucose monitoring on a particular day by the GP's staff (OR 13·6, P<0·001, adjusted OR 8·5, P=0·017). Control measures were implemented. Serological controls after 6 and 18 months revealed that the outbreak was brought under control
INTRODUCTION
The elderly are usually not considered a primary risk group for acquiring infection with hepatitis B virus (HBV). Nonetheless, in addition to sexual contact and drug injection, nosocomial transmission should not be neglected as a risk factor, even in countries with high hygiene standards. In Germany, the exact incidence of nosocomial HBV transmissions is unknown, but various measures have been implemented in recent years to reduce nosocomial HBV infections, e.g. improved hygiene, increased vaccine coverage, increased awareness of medical staff, and highly sensitive testing of blood products [1]. On the other hand the number of invasive diagnostic and therapeutic procedures is increasing [2]. These procedures are also finding increased use in nursing homes, in which a growing proportion of the population is cared for. In this context we report on a large outbreak of HBV infections in a nursing home in Lower Saxony, Germany
In the nursing home, 160 residents were cared for by about 100 employees. The nursing home was divided into four care units (CUs) that had strictly separate staff and care tools. Some of the CUs were further divided into 2 or 3 wards. Medical care of residents was provided by local general practitioners (GPs). The residents had free choice of their GP, but two thirds of the residents had the same GP, henceforth denoted as GP A
A resident of CU II was admitted to the hospital on 10 May 2001 because of poor physical health, and on 22 May was serologically diagnosed with acute hepatitis B. In the resident's ward, contact investigations among residents and staff were conducted. On 22 June a resident of the gerontopsychiatric CU III became icteric and was admitted to the hospital. Acute hepatitis B was diagnosed in this patient on 29 June and contact investigations were also conducted in CU III. Contact investigations for both cases revealed nine further acute infections among residents but no acute or persistent HBV infection in staff members. Thereupon, a decision was made to take the following actions:
Perform a serological investigation of the HBV status of all residents of the nursing home
Perform an epidemiological analysis of risk factors by means of resident's care records in order to identify the route of transmission
Vaccinate susceptible residents
Vaccinate the entire staff
METHODS
Serological assays
Diagnosis of HBV infection was performed stepwise beginning with hepatitis B surface antigen (HBsAg) and antibodies to hepatitis B core antigen (anti-HBc). According to the results further assays of anti-HBc-IgM, anti-HBs, HBeAg and anti-HBe were performed according to the scheme shown in Table 1, rows 1–9. Serological markers of HBV were detected by microparticle enzyme immunoassay (MEIA) using commercially available kits (Abbott, Wiesbaden, Germany). Tests were carried out by an automated analyser (AxSYM, Abott)
Table 1.
Patterns of HBV parameters in 188 residents of a nursing home where an outbreak of acute hepatitis B occurred. If more than one sample was analysed for a resident, the most meaningful result is considered. Only pattern no. 10 is based on two samples indicating seroconversion. More ambiguous patterns due to borderline test results are presented in the bottom of the table. Pattern nos. 1–9 also reflect the procedure of stepwise HBV diagnosis
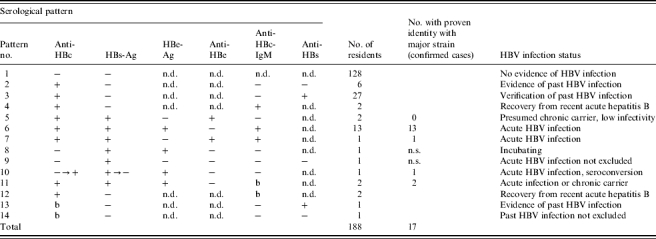
Borderline test result; n.d., test not done; n.s., sequence analysis not successful
Analysis of HBV DNA
HBV DNA was detected by quantitative real-time PCR in a LightCycler (Roche, Mannheim, Germany) using specific primers and probes covering the X gene of the HBV genome from base 1413 to 1601 [3]. Results were expressed as genome molecules (ge)/ml and calibrated using the International Standard preparation for HBV DNA of the WHO [4]. One international unit of this standard corresponds to 5·4 ge according to absolute calibrations [5]. Furthermore a 336 base segment of the S gene region from base 422 to 758 was amplified using real-time PCR [6]. Reagent preparation, extraction of HBV DNA, amplification and analysis of the amplified product were all performed in different rooms to prevent cross contamination. The run control for the PCR had genotype D, the sequence of which differed from the sequences obtained in this study. DNA products of the PCRs were gel-purified and submitted to direct sequencing in both directions (MWG, Ebersberg, Germany). Sequences were aligned and compared using the program Lasergene 6 (DNA Star, Madison, WI, USA), subprograms Editseq for data handling and MegAlign and mega3 (www.megasoftware.net/mega3) for generation of the phylogenetic tree. Confirmation of sequence identity or non-identity was accepted only if both sequence directions yielded the same result. The sequences were compared with various genotypes of HBV using GenBank data
Case-control study
A case-control study was conducted to identify associations between potential risk factors and HBV infection. Serological results provided different strengths of evidence of as to whether an HBV infection had recently occurred and whether infections in different persons were caused by the same virus strain. Therefore, two case definitions were used, one with confirmed strain identity and the second without confirmed strain identity
Confirmed cases. Residents positive for HBsAg and with a DNA sequence indistinguishable from the implicated HBV strain
Probable cases. Residents negative for HBsAg, but positive for anti-HBc-IgM as a marker of recent infection, and without HBV DNA sequence data
Non-infected residents serving as controls. Residents without any sign of acute or past HBV infection, which were neither confirmed nor probable cases and were susceptible for HBV infection
Only those residents were included in the case-control study who lived in affected CUs II and III. Residents who were admitted to these CUs later than 31 May 2001 were excluded. The rationale for choosing this date was that the last admission of a confirmed case was on 29 May 2001. The residents were categorized into cases and controls according to the diagnosis of the serological tests that were conducted for all residents in the course of contact investigations beginning on 1 July 2001. All potential control persons were included, no matching was applied
Data collection
A standardized questionnaire was used to obtain information on risk factors during the period from January to June 2001. The data were predominantly collected from the care records at the nursing home and included information on age, sex, residence in nursing home (admission date, care unit, room-mates), consultations of physicians and hospital admissions, health status (e.g. mobility, care level, diabetes mellitus), invasive and medical procedures (e.g. endoscopies, capillary blood sampling, insulin injection), care procedures, and accidents or injuries. In addition, the questionnaires were partly completed by the responsible GPs, who provided information on HBV vaccination status and potential clinical symptoms of HBV infection. Information on pedicure and haircut were obtained from the corresponding receipts
Statistical analysis
Associations between risk factors and HBV status were determined by logistic regression analysis using stata software (StataCorp, College Station, TX, USA). Multivariate analysis was performed to account for confounding. The analysis was performed for confirmed as well as jointly for confirmed and probable cases. The results were presented by means of odds ratios (OR) accompanied by two-sided 95% confidence intervals (CI) and P values for a two-sided Wald test
RESULTS
Virological results
Between July 2001 and February 2002, 188 residents were tested for serological markers of HBV infection. The results of the successive diagnostic tests are summarized in Table 1. A relatively large proportion of the residents (33/188, 17·5%) had markers indicating a previous HBV infection. Anti-HBc and anti-HBs as signs of naturally acquired immunity were present in 27 residents, six had only anti-HBc. For those 23 individuals who tested positive for HBsAg or anti-HBc-IgM detailed results are given in the line-listing (Table 2). In most cases the tests allowed for a clear diagnosis (Table 1, pattern nos. 1–10), but in some cases borderline results led to equivocal conclusions (Table 1, pattern nos. 11–14). From 21 residents at least one serum sample was obtained that contained HBsAg. These samples were analysed further by DNA sequencing (Table 1, pattern nos. 5–11). In 19 cases sequencing revealed clear results either for both analysed gene regions or for at least one region
Table 2.
Personal data, serological results and clinical features of 23 nursing home residents with markers of HBV infection (line-listing)
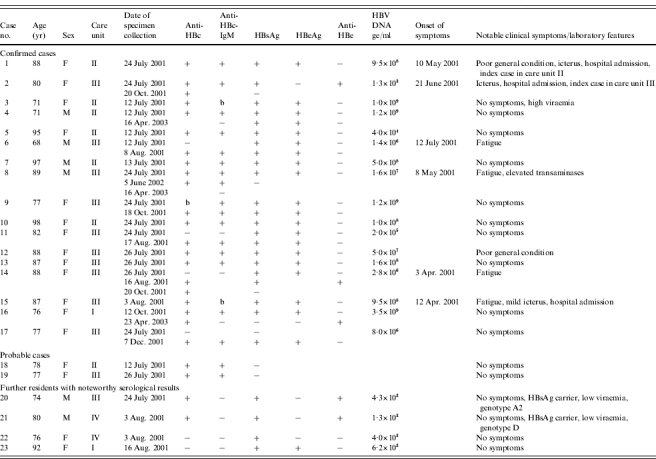
F, Female; M, male; b, borderline result; ge, genome equivalents
In 17 cases HBV genotype A2 was found according to the genosubtyping scheme of Norder et al. [7] with identical DNA sequences, and thus they were classified as confirmed cases belonging most probably to one chain of infection (Table 1, pattern nos. 6, 7, 10 and 11; Table 2 case nos. 1–17). Among these was a woman who was asymptomatic, HBsAg, HBeAg and anti-HBc total antibody-seropositive, of borderline anti-HBc-IgM seropositivity, and highly viraemic (109 ge/ml; Table 2, case no. 3). It was considered that this person who might have been chronically infected, possibly before the outbreak, could have been the source of the outbreak
Fourteen residents with the same HBV DNA sequence were anti-HBc-IgM positive. In case no. 14 (see also Table 1, pattern no. 10) only seroconversion of anti-HBc could be documented. In case no. 15 anti-HBc-IgM was only borderline positive as in case no. 3 (Table 1, pattern no. 11), but this patient has had clinical symptoms of acute hepatitis. Two further residents tested negative for HBsAg, but positive for anti-HBc-IgM (Table 2, case nos. 18 and 19; Table 1, pattern no. 4) and were classified as probable cases
One resident had HBsAg, no anti-HBc and a small amount of HBV DNA whereby sequence analysis was not reliable (Table 2, pattern no. 22). The missing anti-HBc suggested that this was also a recent infection, but occurred in a different CU than the other cases. Thus, the source of her infection was initially considered as questionable. Also another resident (no. 23) did not fulfil the case definition, because she was not admitted to the nursing home until 4 August 2001 and also was not cared for in one of the affected CUs. Shortly after admission, however, this resident was diagnosed with an acute HBV infection. Because of the poor condition of several blood samples a sequence analysis was not possible. It cannot be excluded that this infection was acquired in the nursing home, but due to the distance in time and place from the other infections and the incomplete diagnostic information she was not considered a confirmed or probable case
Two residents tested positive for HBsAg, but were not classified as confirmed cases (Table 2, case nos. 20 and 21; Table 1, pattern no. 5). In one of these cases the HBV genotype was A2 as in the confirmed cases, but the S gene contained one base exchange at position 533 leading to an S protein mutation from methionine 134 to isoleucine. Furthermore, this case (no. 20) had no symptoms of hepatitis, no anti-HBc-IgM, and was anti-HBc and anti-HBe positive with a relatively low viraemia of 430 ge/ml. Thus, it was concluded that this was a chronic carrier with low infectivity who was already infected before the outbreak and not related to the other cases with genotype A2. A further HBsAg-positive resident (no. 21) also had serological markers which are typical for a chronic HBsAg carrier, but he had genotype D and 1300 ge/ml
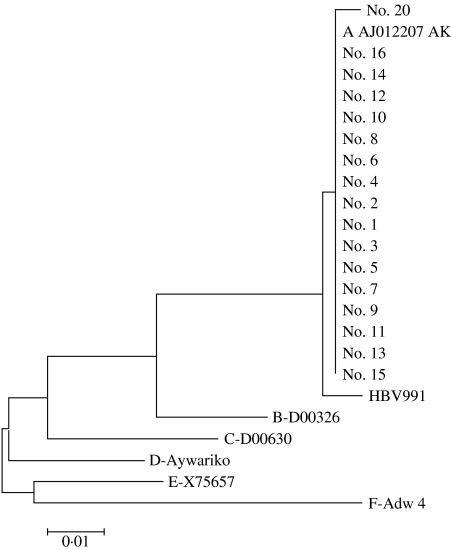
The phylogenetic tree of the sequenced HBV DNA isolates from the 17 residents is shown in the Figure. The confirmed cases had an identical S gene sequence and they all had the sequence of the HBV genotype A2 strain predominating in Germany (EMBL accession no. AJ012207 AK). Other genotype A2 isolates obtained from the GenBank differed in that region by only one or two bases. In contrast, the other HBV genotypes show much larger sequence variations (Fig). Systematic studies of the genotype prevalence and incidence in Germany have not been made. However, the Institute of Medical Virology in Giessen (WHG) has determined the HBV genotype in 80 mostly unrelated HBV cases in Germany in recent years: 28 were of genotype A2 and the same sequence as the 17 patients of this study, 2 were A1 (the African/Asian subgroup of A), 41 genotype D, 1 genotype B, 5 genotype C, 1 genotype E and 1 genotype G. It should be noted that most non-A2 genotypes came from patients of non-German ethnic background
Fig.
Phylogenetic tree of the S gene nucleotide sequences obtained from 17 residents with HBV genotype A2 infection. Patient no. 20 showed one exchange and was probably a chronic asymptomatic carrier. HBV991 is a genotype A2 strain which was cloned in 1980 in Germany with two base exchanges. Genotype A AJ012207AK is the strain from which the WHO International Standard has been derived. The sequences with other genotypes were taken from the gene bank
Epidemiology
As can be seen from the line-listing of the 19 confirmed or probable cases, 11 were residents of CU III and seven were from CU II (Table 2, case nos. 1–19). One confirmed case lived in CU I, but had moved from CU III on 25 May 2001 (no. 16). Thus, the epidemiological investigations were focused on CUs II and III with 67 residents, on which the epidemiological data were collected. Nine of these residents were excluded from the analysis since they were admitted to the nursing home later than 31 May 2001. Another 13 residents were excluded, since they were seropositive for anti-HBc total antibody and seronegative for anti-HBc-IgM, indicating that they were not susceptible to HBV infection, nor were they acutely infected. Thus, 45 residents remained available for epidemiological analysis in this study, 17 of which were classified as confirmed cases, two as probable cases, and 26 as controls
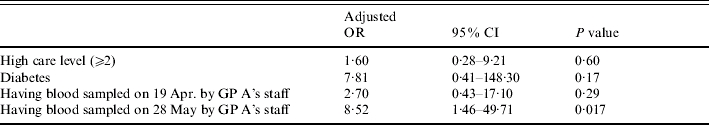
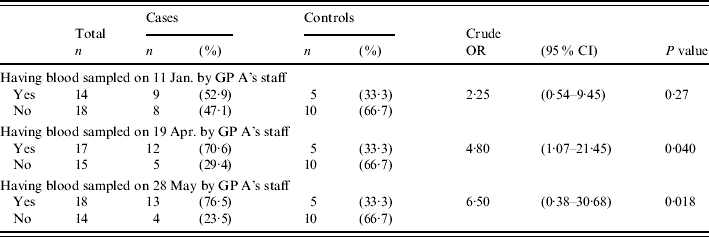
In Tables 3–5 results of the case-control study for confirmed cases are presented. Some risk factors were reported so rarely, that they could not have served as a major transmission route and thus are not presented here. The most significant risk factor was medical care received from GP A (Table 3). All confirmed cases but less than 60% of control subjects had received medical care from this GP. GP A regularly sampled blood from most of his patients for glucose monitoring. The blood was taken by the GP's staff in the residents' rooms. A significant association of infection was found with blood sampling on three days: 11 January, 19 April and 28 May, most significant on the latter two days. The risk associated with blood sampling only applied to residents who received medical care from GP A, which also was a significant risk factor by itself. In order to test whether the significance of blood sampling was not merely a result of receiving care from this GP, further analyses were conducted. Table 4 shows results of the analysis of blood sampling restricted to the subgroup of GP A's patients. Within this subgroup significance vanishes for 11 January. But blood sampling on 19 April and 28 May remained significant risk factors. Thus the association of infection with blood sampling on these two days cannot be explained by confounding due to GP A. Table 5 presents results of the multivariate analysis with the four significant or borderline significant risk factors: high care level (⩾2), diabetes, blood sampling on 19 April, and blood sampling on 28 May. In this analysis only blood sampling on 28 May remained significant
Table 3.
Results of case-control study, confirmed HBV cases vs HBV-negative controls
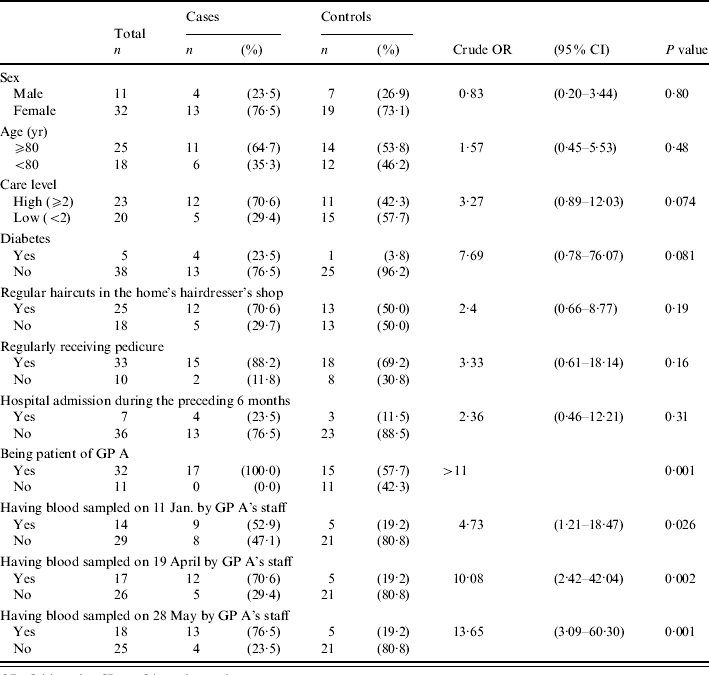
OR, Odds ratio; CI, confidence interval
Table 5.
Results of case-control study, confirmed HBV cases vs. HBV-negative controls, adjusted ORs calculated by multiple logistic regression analysis
OR, Odds ratio; CI, confidence interval
Table 4.
Results of case-control study, confirmed HBV cases vs. HBV-negative controls in the subgroup of residents who are patients of GP A
OR, Odds ratio; CI, confidence interval
When analogous analysis was performed including the two probable cases (case definition 2), with the same set of controls, the association of infection with GP A and blood sampling on 28 May was even more significant. Remarkably, the questionable case (no. 22) had blood taken on 28 May by the staff of GP A. Thus, in retrospect there is similar evidence as for the probable cases, that this case is related to the outbreak
However, the outbreak cannot totally be explained by capillary-blood sampling on one single day. While blood sampling on 28 May showed the strongest association with the outbreak, the index patient no. 1 must have been infected before her hospital admission on 10 May. Another confirmed case was not admitted to the nursing home until 29 May and for this case no capillary blood sampling was documented (no. 17). A further confirmed case also did not take part in any of the three blood samplings mentioned above (no. 9). Case no. 23 with an acute infection was not a patient of GP A. Although a staff member of GP A took blood samples from that person in the course of contact investigations on 14 August, this blood sampling cannot have caused transmission, because this serum was already positive for HBsAg and HBeAg. Sequence analysis failed because several blood samples were haemolytic
Transmission by capillary blood sampling
The procedure of capillary blood sampling was reported by GP A's assistant as follows: Regular blood samples were taken from residents suffering from diabetes, and for prophylactic reasons also from residents not suffering from diabetes. Blood samples were collected mainly in the residents' rooms. The assistant reported that she usually visited the CUs in the order IV/II/I/III, but not all on the same day. However, according to reports of the nursing home's staff the order IV/III/II/I seemed to have occurred. In this case the affected CUs would have been visited successively
Blood sampling was done by puncture at the fingertip or at the ear lobe. First a cotton swab was moistened with 70% isopropyl alcohol from an alcohol dispenser by pressing the swab on the bowl-shaped outlet device. The swab was used to disinfect the skin. For puncture single packed lancets were used. After puncture, the lancet was dropped into a disposal container. A drop of blood was collected by a glass capillary tube and then dropped into a specimen container. The capillary tubes were taken from a storage container and after use dropped into a separate disposal container. Finally the puncture site was covered with an adhesive tape
The following items were carried on one tray: storage container with lancets, storage container with capillary tubes, disposal container for lancets, disposal container for capillary tubes, basket for swabs, basket for adhesive tapes, alcohol dispenser, box for gloves, box for specimen containers. GP A's staff members reported that gloves were used while collecting blood samples. The gloves were, however, not changed for each patient. The alcohol dispenser was disinfected once a week
Control measures
The following measures were established: The indication for regular glucose monitoring was defined much more stringently. Consequently, substantially fewer residents had to be monitored. These patients were sampled by GP A himself. In the case of punctures, the skin was not disinfected. Gloves were used for activities where contact with blood was possible and were changed after each patient
Serological tests that were conducted between November 2001 and April 2002 and between January and March 2003 in 39 initially seronegative residents of CUs II and III did not reveal any further seroconversions
DISCUSSION
Prevalence and incidence of HBV infections in Germany
Anti-HBc as the most universal marker of previous or ongoing HBV infection is present in 8·7% of the German population. The prevalence increases with age to 15% in the >65 years old group. The majority of all anti-HBc-positive persons are also anti-HBs positive (6·7%) as a marker of immunity, but 0·6% have HBsAg as a marker of active infection [8]. The 17·5% prevalence of previous HBV infections in the nursing home was comparable to the general prevalence in elderly people. The proportion of three HBsAg carriers (1·6%) is not significantly higher than in the general population, but the incidence of 19 acute HBV infections within a few months exceeds by far the low incidence in Germany of six notified cases in 100 000 persons per year
Source of infection
The serological investigation revealed 20 confirmed or presumed cases of acute or recent infection and three presumed HBsAg carriers. In one of the acute cases (no. 23) the connection to the outbreak is unclear. In the other 19 cases a common source or a chain of transmission events has to be assumed. In 16 cases, the sequence of the S and/or the X gene was identical with that of one HBsAg carrier among the residents. This carrier had HBeAg and a high viraemia as one of the preconditions for high infectivity. The other two carriers had anti-HBe, low viraemia and, furthermore, different sequences. The absence of anti-HBc-IgM and of clinical or biochemical signs of liver damage makes it very unlikely that these two persons were recently infected. Low viraemia is consistent with the assumption that these two carriers were not the source of the outbreak. The one base exchange in the carrier with genotype A2 (patient no. 20) is probably significant, because the amplification protocol used in this study generates very little variability and all A2 sequences from Germany obtained recently in our laboratory were identical except for that one carrier. However, sequence identity is not a proof of a common source in this outbreak, because the involved HBV strain is very common in Germany. In fact an isolate with exactly the same sequence served as starting material for the Eurohep Reference sample [5] and for the WHO International Standard [4]. An identical sequence was found in a recent study from The Netherlands [9] and in an HBV transmission event by a blood transfusion in Germany [10]. The high degree of identity among genotype A2 isolates in Germany has previously been reported [11]. Thus, the sequence identity in the majority of the cases proved only that a common source was possible. However, the occurrence of the symptomatic infections and the presence of anti-HBc-IgM in 16 cases provided evidence for the time course of transmission. Three of the confirmed cases were still in the incubation period when first diagnosed. Anti-HBc-IgM turned out to be a very useful marker for recent HBV infection, although some acute cases were either negative (nos. 22 and 23) or borderline (no. 15), whereas the probable source of infection was also borderline positive although an asymptomatic carrier. It is known that during the very early or late phases of acute infection anti-HBc-IgM may be low or even negative whereas some carriers may be weakly positive [12]
Potential modes of transmission
Initially, pedicure was suspected as a risk factor, but it did not show a significant association with the outbreak. In addition, pedicure would not plausibly explain the infections in two CUs which were served by two different pedicurists. However, in the course of the outbreak investigation control procedures for strict compliance of pedicurists with hygienic recommendations were established. Haircutting in the in-house hairdresser's shop also did not show significant association with the infections. In addition, hairdressing would not explain why only certain CUs were affected, since residents from all CUs attended the shop. Care procedures could also be excluded as the transmission route since only very few residents had corresponding risk factors such as open wounds or invasive procedures
In the literature, outbreaks of HBV were mainly reported in two contexts: outbreaks among injecting drug users due to needle sharing, mainly investigated in prison environments [13–15], and nosocomial outbreaks. Nosocomial outbreaks can be subdivided according to the transmission route from medical staff to patient, or from patient to patient [16]. The former is presumed to play a minor role [17]. The latter can occur by unsafe injections [18] or by infusion of blood components which were contaminated during processing, e.g. through dialysis [19] or through so-called autohaemotherapy [1]. A major role is played by puncture with contaminated needles or other devices, e.g. during acupuncture [20, 21], by electrodes [22], or by so-called jet injection [23]. Transmission in connection with glucose monitoring, which has already been reported several times, also falls in this category [24–29]
Risk factor capillary blood sampling
In this outbreak, only patients of GP A had acquired infection in the sense of the case definitions. Capillary-blood collection by GP A's staff was the risk factor with the strongest association for infection. In addition, this transmission mechanism explains why two CUs with completely separate staff and care tools were affected. Among the patients of GP A, those who provided capillary-blood samples on 19 April and 28 May were affected significantly more often. The carrier, who was the suspected source of transmission, was also sampled on these two days. Although in this outbreak some cases could not be explained by improper capillary-blood sampling, statistical significance as well as reports from the literature strongly suggest that this type of blood sampling was the major transmission route
From the report of the sampling procedure by GP A's staff it was concluded that a risk could have resulted from the application of the alcohol dispenser. Since it was located next to the specimen containers, it may have been contaminated by drops of blood. This could have led to contamination of the swabs and thus the skin of the patients with virus-containing blood, which entered the body by puncture. Not changing gloves between patients was a further potential source of infection, particularly if lancets were contaminated by unclean gloves during unpacking. On the basis of the subsequent serological tests and the fact that no further persons developed clinical symptoms we concluded that after establishing the control measures no further infections had occurred and thus the outbreak was brought under control
As in previous studies, our investigation revealed that HBV outbreaks in nursing homes are favoured by two conditions [28–30]. First, HBV infections in elderly persons often proceed without clinical symptoms [29]. Therefore, the outbreak and thus the potential of the HBV-infected residents for virus transmission may remain unapparent for a prolonged period. Second, the nursing home setting is convenient for conducting mass glucose monitoring, although it bears a high risk of patient-to-patient transmission if necessary precautions are not strictly followed [29]. Our recommendations are that glucose monitoring in nursing homes should be reduced to only essential cases. In particular, mass glucose monitoring in which supplies are carried from resident to resident should be avoided [29]. Of course, when taking blood samples, the standard precautions and recommendations must be strictly followed [29]. This requires the reinforcement of these procedures in training programmes for health-care workers and the incorporation of these procedures into institutional policies [30]
ACKNOWLEDGEMENTS
We thank the nursing home for the kind cooperation which made these investigations possible
DECLARATION OF INTEREST
None
REFERENCES
- 1.Webster GJ et al. Molecular epidemiology of a large outbreak of hepatitis B linked to autohaemotherapy. Lancet. 2000;356:379–384. doi: 10.1016/S0140-6736(00)02529-0. [DOI] [PubMed] [Google Scholar]
- 2.Robert Koch-Institut. On the situation concerning important infectious diseases in the year 1999: Part 2: Viral hepatitis [in German] Epidemiologisches Bulletin. 2000;28:223–227. [Google Scholar]
- 3.Jursch CA et al. Molecular approaches to validate disinfectants against hepatitis B virus. Medical Microbiology and Immunology. 2002;190:189–197. doi: 10.1007/s00430-001-0103-0. [DOI] [PubMed] [Google Scholar]
- 4.Saldanha J et al. An international collaborative study to establish a World Health Organization international standard for hepatitis B virus DNA nucleic acid amplification techniques. Vox Sang. 2001;80:63–71. doi: 10.1046/j.1423-0410.2001.00003.x. [DOI] [PubMed] [Google Scholar]
- 5.Heermann KH et al. Quantitative determination of hepatitis B virus DNA in two international reference plasmas. Journal of Clinical Microbiology. 1999;37:68–73. doi: 10.1128/jcm.37.1.68-73.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schaefer S et al. Universal primers for real time amplification of DNA from all known orthohepadnavirus species. Journal of Clinical Virology. 2003;27:30–37. doi: 10.1016/s1386-6532(02)00108-7. [DOI] [PubMed] [Google Scholar]
- 7.Norder H et al. Genetic diversity of hepatitis B virus strains derived worldwide: genotypes, subgenotypes, and HBsAg subtypes. Intervirology. 2004;47:289–309. doi: 10.1159/000080872. [DOI] [PubMed] [Google Scholar]
- 8.Robert Koch-Institut. On the situation concerning important infectious diseases in Germany. Viral hepatitis B and C in the year 2003 [in German] Epidemiologisches Bulletin. 2004;37:307–315. [Google Scholar]
- 9.Koppelman MH, Zaaijer HL. Diversity and origin of hepatitis B virus in Dutch blood donors. Journal of Medical Virology. 2004;73:29–32. doi: 10.1002/jmv.20057. [DOI] [PubMed] [Google Scholar]
- 10.Meisel H et al. Transmission of hepatitis B virus two months prior to HBsAg positivity of donor blood. Transfusion Medicine and Hemotherapy. 2003;30:228–231. [Google Scholar]
- 11.Uy A et al. Genomic variability in the preS1 region and determination of transmission routes of hepatitis B virus. Journal of General Virology. 1992;73:3005–3009. doi: 10.1099/0022-1317-73-11-3005. [DOI] [PubMed] [Google Scholar]
- 12.Gerlich WH et al. Cutoff levels of immunoglobulin M antibody against viral core antigen for differentiation of acute, chronic and past hepatitis B virus infections. Journal of Clinical Microbiology. 1986;24:288–293. doi: 10.1128/jcm.24.2.288-293.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Love A, Stanzeit B. An epidemic of hepatitis B virus infection among intravenous drug users in Iceland. European Journal of Epidemiology. 1995;11:397–402. doi: 10.1007/BF01721224. [DOI] [PubMed] [Google Scholar]
- 14.Hutchinson SJ et al. Hepatitis B outbreak at Glenochil prison during January to June 1993. Epidemiology and Infection. 1998;121:185–191. doi: 10.1017/s0950268898001022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stevenson J et al. An outbreak of acute hepatitis B infection among injecting drug users in Inverclyde, Scotland. Communicable Disease and Public Health. 2001;4:60–63. [PubMed] [Google Scholar]
- 16.Hasselhorn HM, Hofmann F. Nosocomial hepatitis B virus, hepatitis C virus and HIV infections by infectious medical personnel. Gesundheitswesen. 1998;60:545–551. [PubMed] [Google Scholar]
- 17.Petrosillo N et al. Molecular epidemiology of an outbreak of fulminant hepatitis B. Journal of Clinical Microbiology. 2000;38:2975–2981. doi: 10.1128/jcm.38.8.2975-2981.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.CDC. Transmission of hepatitis B and C viruses in outpatient settings – New York, Oklahoma, and Nebraska, 2000–2002. Morbidity and Mortality Weekly Report. 2003;52:901–906. [PubMed] [Google Scholar]
- 19.Hutin YJ et al. An outbreak of hospital-acquired hepatitis B virus infection among patients receiving chronic hemodialysis. Infection Control and Hospital Epidemiology. 1999;20:731–735. doi: 10.1086/501573. [DOI] [PubMed] [Google Scholar]
- 20.Slater PE et al. An acupuncture-associated outbreak of hepatitis B in Jerusalem. European Journal of Epidemiology. 1988;4:322–325. doi: 10.1007/BF00148918. [DOI] [PubMed] [Google Scholar]
- 21.Walsh B et al. Outbreak of hepatitis B in an acupuncture clinic. Communicable Disease and Public Health. 1999;2:137–140. [PubMed] [Google Scholar]
- 22.Hepatitis B Outbreak Investigation Team. An outbreak of hepatitis B associated with reusable subdermal electroencephalogram electrodes. Canadian Medical Association Journal. 2000;162:1127–1131. [PMC free article] [PubMed] [Google Scholar]
- 23.Canter J et al. An outbreak of hepatitis B associated with jet injections in a weight reduction clinic. Archives of Internal Medicine. 1990;150:1923–1927. [PubMed] [Google Scholar]
- 24.Douvin C et al. An outbreak of hepatitis B in an endocrinology unit traced to a capillary-blood-sampling device. New England Journal of Medicine. 1990;322:57–58. doi: 10.1056/NEJM199001043220112. [DOI] [PubMed] [Google Scholar]
- 25.Panknin HT. Nosocomial hepatitis B in capillary blood collection for blood sugar determination by reflectometric interpretation. Krankenpflege Journal. 1993;31:250–252. [PubMed] [Google Scholar]
- 26.CDC. Nosocomial hepatitis B virus infection associated with reusable fingerstick blood sampling devices – Ohio and New York City, 1996. Morbidity and Mortality Weekly Report. 1997;46:217–221. [PubMed] [Google Scholar]
- 27.Quale JM et al. Deja vu: nosocomial hepatitis B virus transmission and fingerstick monitoring. American Journal of Medicine. 1998;105:296–301. doi: 10.1016/s0002-9343(98)00256-3. [DOI] [PubMed] [Google Scholar]
- 28.De Schrijver K 2005. http://www.eurosurveillance.org/ http://www.eurosurveillance.org/ , Eurosurveillance Editorial Team. Hepatitis B transmission in care homes linked to blood glucose monitoring, Belgium and United States. Eurosurveillance Weekly . ; 17 March: 10 (
- 29.CDC. Transmission of hepatitis B virus among persons undergoing blood glucose monitoring in long-term care facilities, Mississippi, North Carolina, and Los Angeles county, California, 2003–2004. Morbidity and Mortality Weekly Report. 2005;54:220–223. [PubMed] [Google Scholar]
- 30.Williams IT et al. Viral hepatitis transmission in ambulatory health care settings. Clinical and Infectious Diseases. 2004;38:1592–1598. doi: 10.1086/420935. [DOI] [PubMed] [Google Scholar]