SUMMARY
Stringency of milk pasteurization has been established on requirements for Coxiella burnetii as being the most heat-resistant organisms of public heath significance. This paper discusses the estimation of the efficiency of pasteurization time/temperature combinations as required in regulations for food safety. Epidemiological studies have been interpreted as C. burnetii being a significant pathogen causing clinical disease through ingestion of milk. The paper examines the evidence and challenges the designation of C. burnetii as a foodborne pathogen. Consequently it questions the need for pasteurization parameters to be established on its heat resistance characteristics.
INTRODUCTION
Milk pasteurization was introduced to prevent the oral transmission of tuberculosis, brucellosis, and other milk-borne infectious diseases. Early in the twentieth century, it was established that the cells of the tubercle bacillus were the most heat-resistant vegetative bacterial cells in milk. Therefore, the first recommendations for time and temperature combinations for pasteurization were established on this basis. However, pasteurization of milk is defined by the Codex alimentarius Committee for Food Hygiene [1] as ‘a microbiocidal heat treatment aimed at reducing the number of any pathogenic microorganisms in milk and liquid milk products, if present, to a level at which they do not constitute a significant health hazard. Pasteurization conditions are designed to effectively destroy the organisms Mycobacterium tuberculosis and Coxiella burnetii’. Thus the international definition points to the need for the destruction of Coxiella burnetii to protect the health of milk consumers.
C. burnetii is the cause of Q fever, recognized in 1935 as an occupational disease of workers in abattoirs in Australia and as a tick-transmitted disease in the United States [2]. After the Second World War, a high prevalence of Q fever and serological conversion was observed among the population in Europe and North America, in regions where raw milk and raw milk products were commonly consumed [3, 4]. There was a consensus that milk should not be consumed raw and, therefore, milk pasteurization was recommended. Studies were conducted in several countries to check the efficiency of heat against C. burnetii [3, 5–9]. Eventually time-temperature conditions for pasteurization published by US researchers in 1957 [10–12] became the international standard.
In the first part of this paper, we will indicate how these researchers used a safety factor, and will show that the recommended heating treatment not only provides at least 4·7 decimal reductions or ‘log kills’ (rather than 5 as usually reported), but plausibly even more. In the second part we will question if Q fever is a foodborne disease and if pasteurization is scientifically justified for the prevention of Q fever.
Heat resistance of C. burnetii
A number of studies conducted to measure the heat resistance of C. burnetii did not lead to any convincing conclusion related to the efficacy of time-temperature combinations used in pasteurizers [3, 5–9]. Enright et al. [11, 12] put an end to this by publishing undisputed results validated through infection studies in guinea pigs by intraperitoneal inoculation. Since the appearance of specific complement-fixing antibody was significantly induced by killed C. burnetii, the authors demonstrated the presence of infective viable microbial cells by two consecutive passages on guinea pigs. They used whole raw milk from an experimentally infected cow containing 105 infecting doses in 2 ml (5×104 infective doses/ml) and heated in the laboratory at temperatures from 60·6 to 66·1°C for different lengths of time.
For the shortest heating times, viable cells were still present. For the longest heating times, no viable cells could be found. Linear regressions of log10(time) against temperature were calculated to determine two lines:
the line A below which vials were still positive (containing at least one surviving cell);
the line B over which no vial contained survivors (corresponding to the ‘minimum time of destruction’ according to the authors).
Positive as well as negative vials could be found between lines A and B.
A second series of experiments conducted by a regular commercial pasteurization plant from 68·1 to 72·8°C confirmed the validity of the first series.
The authors based their recommendations for pasteurization conditions by adding two standard deviations or 97·7% confidence interval to the minimum times of destruction estimated by the regression B. They finally recommended two time-temperature combinations that have subsequently been universally recognized: 30 min at 62·8°C (145 F) or 15 s at 71·7°C (161 F).
The influence of temperature is, therefore, given by z=4·34°C. These recommendations were then simplified as follows [1, 13]: 30 min at 63°C or 15 s at 72°C, thus providing an extra safety margin. Assuming the survival curves are straight lines, this would achieve eight decimal reductions.
If, for a given temperature log10(a) is the ordinate of line A, and log10(b) the ordinate of line B, then the most probable time t for which there is one survivor per vial is [14]:
and the decimal reduction time is calculated with [14]:
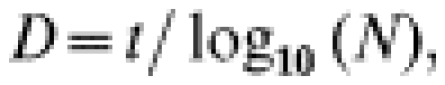 |
where N is the initial number of microbial cells per vial.
The experimental data of Enright et al. [11] are reported in the Table together with calculated D values and numbers of decimal reductions corresponding to recommended treatments.
Table.
Number of decimal reductions (log kills) of C. burnetii demonstrated experimentally, and values calculated for internationally recommended pasteurization time/temperature combinations, assuming a linear survival curve. Calculations were done with z=4·34°C
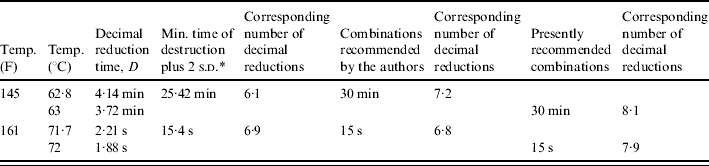
s.d., Standard deviation.
The experimental work of Enright et al. [11, 12] was performed carefully. It was the first study regarding C. burnetii where the results were modelled using statistical regression, and where a safety margin was used. However, the paper was not clear as to the origin of the C. burnetii cells subjected to heating: whether they were from one or several animals; and whether a single strain or a mixture of strains was used? The shape of survival curves was not studied, and it was not checked if the curve was linear or biphasic, i.e. having a second part or ‘tail’ indicative of a slower killing rate; therefore, the addition of two standard deviations by Enright et al. [11] did not guarantee a larger killing effect. While there is no certainty about the actual number of decimal reductions, one can nevertheless reasonably assume that, for the studied strain(s), pasteurization achieved between 4·7 [i.e. log log10(5×104)] and 8 decimal reductions of C. burnetii.
Transmission of Q fever to people
It is well documented that C. burnetii is transmitted to man from infected wild or domesticated mammals, including farm animals and pets, by inhalation and by bites of haematophagous arthropods. The disease affects mostly farmers, veterinarians, researchers, abattoir workers and persons exposed to aerosols in dwellings situated down wind, or being in the vicinity of infected herds [15–49]. All outbreaks and sporadic cases reported for the last 50 years in Australia, France, Germany, Italy, and the United States were attributed to inhalation and sometimes to arthropod bites [23, 24, 26, 27, 31, 34, 36, 39, 47, 50–55]. No information is given of the infective dose.
In his comprehensive review, Wegener [3] noted the widespread opinion at that time: ‘Milk is the most significant source among products of animal origin. Personnel in dairies and their families with the greatest use of raw milk are heavily infected in the regions with Q fever problems in North America and Italy.’ This opinion on foodborne transmission was based on a survey in the United States where 10·7% of people consuming raw milk had a positive serological test, compared to 0·7% among non-exposed people. Other authors used the same argument on the basis of observations in England [4, 17, 56] and in other countries [18, 19, 25, 57–60]. Nevertheless several reports mentioned the oral route as possible but infrequent, circumstantial, or needing a very high dose [15, 33, 44, 61–63]. According to Enright et al. [11], milk of naturally infected cows contained the following numbers of guinea pig infective doses (ID) per millilitre: 1 (5 animals), 10 (5 animals), 100 (5 animals) or 1000 (3 animals), while the milk of an experimentally infected cow contained 10 000 guinea pig ID/ml. These microbiological loads should be compared to those of inhaled air around infected animals or herds. However, we could not find any indication of these.
A few publications that reported a correct epidemiological approach did confirm that seroconversion indicated infection, but not the clinical disease. Fishbein et al. [18] reported a significant association between seropositivity and drinking non-pasteurized milk products whether people were in contact with goats or not, but the article did not provide information about clinical disease. Benson et al. [62] indicated that 35% of prisoners drinking infected milk had a positive serological test against 4% in a non-exposed control group; yet the authors emphasized that no manifestations of disease were recorded. Hatchette et al. studied an outbreak affecting goats in Newfoundland (Canada):
Risk factors associated with human infection [based on people with serological conversion, but where infection was not confirmed and no indication was given in the paper about manifestation of disease] on univariate analysis included being a farmer, milking goats, assisting with kidding, handling placentas, shovelling manure, having direct contact with goats, eating cheese made from goat milk, petting goats, feeding goats, being a worker, smoking tobacco, and drinking alcohol. When only a multivariate analysis was used, the following were significant risk factors for infection with C. burnetii: contact with the placenta (P<0·001), smoking history (P=0·001), and eating cheese made from [pasteurized] goat milk (P=0·022). Consumption of goat milk itself was not associated with an increased risk of infection (OR 1·07) [30].
The authors concluded: ‘The reason for the association between ingesting goat cheese and developing Q fever is not clear and suggests further study is needed. At present, this is an epidemiological association only, as C. burnetii has not been recovered from the goat cheese.'
There is also evidence from Australia that indicates direct contact (inhalation) with C. burnetii is more important in causing Q fever than other exposure including ingestion, as detected by immunological reactivity. Q fever has been a notifiable disease in Australia since 1977 with about 600 cases reported each year (range 202–870) [64, 65]. The majority of notified cases (60%) are from people employed in the meat industry as abattoir workers. About 30% of notified cases of Q fever are from the agricultural industry [64]. This would represent a Q fever notification rate of 1240/100 000 and 164/100 000 for meat and agricultural sectors respectively. In contrast, immunological reactivity is more commonly found in the agricultural rather than the meat industry.
In Australia, immunological testing of people presenting for Q fever vaccination programmes for people at risk of infection in meat works or rural communities shows that prior to vaccination 17% of meat industry workers compared to 28% from the agricultural industries (including farm families) had positive reactions indicating previous exposure to C. burnetii [65]. All cows' milk sold in Australia must be pasteurized in accordance with Food Standards regulations and non-pasteurized milk is only available to farming families. It is also of interest to note that 85% of notified cases of Q fever in Australia are males and 70% of cases are between 20 and 50 years [65]. This pattern of disease is different from potential exposure through consumption of non-pasteurized milk.
Some authors accepting the foodborne transmission paradigm assumed that the form of the disease could be different according to the route of contamination: hepatitis for ingestion, pneumonia for inhalation [18, 28, 58, 66–70]. It is now recognized that this is not true [71].
CONCLUSION
From what is reported above, it seems more than plausible that clinical disease of Q fever results only from inhalation of C. burnetii and sometimes arthropods bites. Ingestion of C. burnetii-contaminated milk or milk products may result in serological conversion potentially indicating infection but not necessarily clinical disease. In addition it is likely that seroconversion follows the ingestion of inactivated cells as well as of live cells. Therefore, one may question:
Should Q fever be still listed among the foodborne zoonoses?
Should temperature and time conditions for milk pasteurization still be based on the heat resistance of C. burnetii?
If the answer is ‘no’ to both questions, the historical decision to pasteurize milk in order to kill C. burnetii, made almost 50 years ago, could be considered retrospectively as an early example of the application of the precautionary principle.
ACKNOWLEDGEMENTS
We acknowledge collaboration with A. Mimouni. Part of this work is a contribution by one of us (O.C.) to a report to the French Food Safety Agency (AFSSA).
DECLARATION OF INTEREST
None.
REFERENCES
- 1.Anon 2004. . Code of Hygienic Practice for Milk and Milk Products. Washington DC, USA, 29 March–2 April 2004: Joint FAO/WHO Food Standards Programme – Codex Committee on Food Hygiene, 26th Session,
- 2.Marrie T, Raoult D. Q fever – a review and issues for the next century. International Journal of Antimicrobial Agents. 1997;8:145–161. doi: 10.1016/s0924-8579(96)00369-x. [DOI] [PubMed] [Google Scholar]
- 3.Wegener KH. Q Fever and its signification regarding milk hygiene. Bibliographic study with contribution to the question of its occurrence in cattle herds in Schleswig-Holstein [in German] Kieler Milchwirtschaftliche Forschungsberichte. 1957;9:509–535. [Google Scholar]
- 4.Marmion B, Stoker G. The epidemiology of Q fever in Great Britain. British Medical Journal. 1958;2:809–816. doi: 10.1136/bmj.2.5100.809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Huebner RJ et al. Q fever studies in southern California: III. Effects of pasteurization on survival of C. burnetii in naturally infected milk. Public Health Report. 1949;64:499–511. [PMC free article] [PubMed] [Google Scholar]
- 6.Kirberger E. In vitro resistance of Coxiella burneti [in German] Zeitschrift für Tropenmedizin und Parasitologie. 1951;3:77–86. [PubMed] [Google Scholar]
- 7.Bingel K, Engelhardt H. Are our pasteurization processes sufficent to make innoxious the pathogen of Q fever in cow milk? [in German] Archiv für Hygiene. 1952;136:417–425. [PubMed] [Google Scholar]
- 8.Marmion B et al. The effect of pasteurization on milk containing Rickettsia burneti. Monthly Bulletin of the Ministry of Health and the Public Health Laboratory Service. 1951;10:119–128. [PubMed] [Google Scholar]
- 9.Lennette EH et al. Q fever studies XIII. The effect of pasteurization on Coxiella burnetti in naturally infected milk. American Journal of Hygiene. 1952;55:246–253. [PubMed] [Google Scholar]
- 10.Anon. Q fever and milk pasteurization. Public Health Report. 1957;2:947–948. [PMC free article] [PubMed] [Google Scholar]
- 11.Enright JB, Sadler WW, Thomas RC. Pasteurization of milk containing the organism of Q fever. American Journal of Public Health. 1957;47:695–700. doi: 10.2105/ajph.47.6.695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Enright JB, Sadler WW, Thomas RC Washington, DC: U.S. Government Printing Office; 1957. . Thermal inactivation of Coxiella burnetii and its relation to pasteurization of milk, Public Health Monograph No. 47. PHS Publication No. 517. [Google Scholar]
- 13.Staal P, Cerf O. Bulletin of the International Dairy Federation, Bulletin 200/1986. Brussels: International Dairy Federation; 1986. Introduction. [Google Scholar]
- 14.Cerf O, Federighi M, Tholozan J-L. Traitements ionisants et hautes pressions des aliments. Paris, France: Polytechnica; 2001. Theory of microorganisms inactivation [in French] [Google Scholar]
- 15.Acha PN, Szyfres B Paris: Office international des épizooties; 1989. . Zoonoses and communicable diseases common to man and animals [in French]. [Google Scholar]
- 16.Jorm LR, Lightfoot NF, Morgan KL. An epidemiological study of an outbreak of Q fever in a secondary school. Epidemiology and Infection. 1990;104:467–477. doi: 10.1017/s0950268800047476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Connolly JH et al. Clinical Q fever in Northern Ireland 1962–1989. Ulster Medical Journal. 1990;59:137–144. [PMC free article] [PubMed] [Google Scholar]
- 18.Fishbein DB, Raoult D. A cluster of Coxiella burnetii infections associated with exposure to vaccinated goats and their unpasteurized dairy products. American Journal of Tropical Medicine and Hygiene. 1992;47:35–40. doi: 10.4269/ajtmh.1992.47.35. [DOI] [PubMed] [Google Scholar]
- 19.Brouqui P et al. Chronic Q fever. Ninety-two cases from France, including 27 cases without endocarditis. Archives of Internal Medicine. 1993;153:642–648. doi: 10.1001/archinte.153.5.642. [DOI] [PubMed] [Google Scholar]
- 20.Thomas DR et al. The risk of acquiring Q fever on farms: a seroepidemiological study. Occupational and Environmental Medicine. 1995;52:644–647. doi: 10.1136/oem.52.10.644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Manfredi Selvaggi T et al. Investigation of a Q fever outbreak in Northern Italy. European Journal of Epidemiology. 1996;12:403–408. doi: 10.1007/BF00145305. [DOI] [PubMed] [Google Scholar]
- 22.Pebody RG et al. Epidemiological features of Coxiella burnetii infection in England and Wales: 1984–1994. Communicable Clinical Disease Report. 1996;6:R128–R132. [PubMed] [Google Scholar]
- 23.Armengaud A et al. Urban outbreak of Q fever, Briançon, France, March to June 1996 [in French] Eurosurveillance. 1997;2:12–13. doi: 10.2807/esm.02.02.00137-en. [DOI] [PubMed] [Google Scholar]
- 24.Lyytikäinen O et al. Outbreak of Q fever in Lohra-Rollshausen, Germany, spring 1996 [in German] Eurosurveillance. 1997;2:9–11. doi: 10.2807/esm.02.02.00136-en. [DOI] [PubMed] [Google Scholar]
- 25.Serbezov V et al. Q fever in Bulgaria and Slovakia. Emerging Infectious Clinical Diseases. 1999;5:388–394. doi: 10.3201/eid0503.990309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tissot-Dupont H et al. Hyperendemic focus of Q fever related to sheep and wind. American Journal of Epidemiology. 1999;150:67–74. doi: 10.1093/oxfordjournals.aje.a009920. [DOI] [PubMed] [Google Scholar]
- 27.Baret M et al. Coxiella burnetii pneumopathy on return from French Guiana [in French] Bulletin de la Société de Pathologie Exotique. 2000;93:325–327. [PubMed] [Google Scholar]
- 28.Norlander L. Q fever epidemiology and pathogenesis. Microbes and Infection. 2000;2:417–424. doi: 10.1016/s1286-4579(00)00325-7. [DOI] [PubMed] [Google Scholar]
- 29.Petersen LR et al. Developing national epidemiological capacity to meet the challenges of emerging infections in Germany. Emerging Infectious Clinical Diseases. 2000;6:576–584. doi: 10.3201/eid0606.000605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hatchette TF et al. Goat-associated Q fever: a new clinical disease in Newfoundland. Emerging Infectious Clinical Diseases. 2001;7:413–419. doi: 10.3201/eid0703.010308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hellenbrand W, Breuer T, Petersen L. Changing epidemiology of Q fever in Germany, 1947–1999. Emerging Infectious Clinical Diseases. 2001;7:789–796. doi: 10.3201/eid0705.010504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nebreda T et al. Outbreak of Q fever and seroprevalence in a rural population from Soria Province [in Spanish] Enfermedades Infecciosas y Microbiología Clínica. 2001;19:57–60. doi: 10.1016/s0213-005x(01)72561-x. [DOI] [PubMed] [Google Scholar]
- 33.Rousset E et al. Modalities of Q fever transmission to man [in French] Bulletin épidémiologique, AFSSA. 2003;2003:1–4. [Google Scholar]
- 34.Vincent C, Desjardins F. Q fever [in French] Bulletin zoosanitaire. Épidémiosurveillance Animale. 2001;29:1–4. [Google Scholar]
- 35.Anon. Q fever [in German] Epidemiologisches Bulletin, Robert Koch Institut. 2002;37:313–317. [Google Scholar]
- 36.de Benoist A-C Saint-Maurice: Institut National de Veille Sanitaire; 2002. . Q fever outbreak in the Chamonix Valley, France, summer 2002 [Communiqués de presse]. [Google Scholar]
- 37.Anon. Factsheet: Q fever. New South Wales Public Health Bulletin. 2002;13:191. doi: 10.1071/nb02076. [DOI] [PubMed] [Google Scholar]
- 38.Carrieri M et al. Investigation of a slaughterhouse-related outbreak of Q fever in the French Alps. European Journal of Clinical Microbiology and Infectious Clinical Diseases. 2002;21:17–21. doi: 10.1007/s10096-001-0645-5. [DOI] [PubMed] [Google Scholar]
- 39.Benoist A-Cd, Mailles A, Dennetiere G. Q fever outbreak in the Chamonix Valley, France, summer 2002. Eurosurveillance Weekly. 2002;6 [Google Scholar]
- 40.Cekanac R, Lukac V, Coveljic M. An epidemic of Q fever in a unit of the Yugoslav Army during war conditions [in Serbian] Vojnosanit Pregl. 2002;59:157–160. doi: 10.2298/vsp0202157c. [DOI] [PubMed] [Google Scholar]
- 41.Kotton C http://www.nlm.nih.gov/medlineplus/ency/article/001337.htm#Causes,%20incidence,%20 and%20risk%20factors. http://www.nlm.nih.gov/medlineplus/ency/article/001337.htm#Causes,%20incidence,%20 and%20risk%20factors . MedlinePlus Medical Encyclopedia – Q Fever; 2002 ( ). Accessed 31 Jan. 2006.
- 42.Maltezou H, Raoult D. Q fever in children. Lancet Infectious Clinical Diseases. 2002;2:686–691. doi: 10.1016/s1473-3099(02)00440-1. [DOI] [PubMed] [Google Scholar]
- 43.Stiles ME, Doyle MP. Foodborne Bacterial Pathogens. Weimar, Texas: Culinary and Hospitality Industry Publications Services; 2002. Less recognized or presumptive foodborne pathogenic bacteria. [Google Scholar]
- 44.Anon Atlanta, GA: 2003. . Q fever. Viral and Rickettsial Zoonoses Branch, Center for Clinical Disease Control and Prevention, . Accessed 31 Jan. 2006. [Google Scholar]
- 45.Anon http://www.vetmed.wisc.edu/pbs/zoonoses/Q%20fever/qfvrindx.html. http://www.vetmed.wisc.edu/pbs/zoonoses/Q%20fever/qfvrindx.html . Q fever, 2003 ( ). Accessed 31 Jan. 2006.
- 46.Anon http://www.vet-alfort.fr/ http://www.vet-alfort.fr/ . Fièvre Q. Polycopiés de maladies contagieuses des Écoles vétérinaires françaises (2002–2003), 2003 ( ). Accessed 31 Jan. 2006.
- 47.Rey S Saint-Maurice: Institut National de Veille Sanitaire; 2003. p. 44. . Investigation on a community outbreak of Q fever. Montoison (Drôme) [in French]. , p. [Google Scholar]
- 48.Rousset E et al. Epidemiology of animal Q fever. Situation in France [in French] Médecine des Maladies Infectieuses. 2001;31:233–246. (Suppl. 2): [Google Scholar]
- 49.Weise E. Q-Fieber. Bonn (Deutschland): Bundesinstitut für Risikobewertung; 2003. [Google Scholar]
- 50.Anon 2003. . Medical NBC Online Information Server,
- 51.McQuiston J, Childs J. Q fever in humans and animals in the United States. Vector Borne Zoonotic Disease. 2002;2:179–191. doi: 10.1089/15303660260613747. [DOI] [PubMed] [Google Scholar]
- 52.Anon 2002. . Establishment of Specifications and Standards Based on the Food Sanitation Law – 2002. FFCR – The Japan Food Chemical Research Foundation, Information on Food Safety in Japan, Ministry of Health, Labour and Welfare,
- 53.Mak D, Fry D, Bulsara M. Prevalence of markers of Q fever exposure in the Kimberley, Western Australia. Communicable Clinical Diseases Intelligence. 2003;27:267–271. doi: 10.33321/cdi.2003.27.51. [DOI] [PubMed] [Google Scholar]
- 54.Santoro D et al. Q fever in Como, Northern Italy. http://www.cdc.gov/ncidod/EID/vol1Onol/03-0467.htm. Emerging Infectious Clinical Diseases. 2004;10 doi: 10.3201/eid1001.030467. ). Accessed 1 Feb. 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Smith R. Occupational exposure risk for Q fever and other zoonoses among those working on control of the foot and mouth clinical disease epidemic in the United Kingdom. http://www.eurosurveillance.org/ew/2001/010705.asp#1. Eurosurveillance Weekly. 2001;7 ). Accessed 31 Jan. 2006. [Google Scholar]
- 56.Brown G, Colwell D, Hooper W. An outbreak of Q fever in Staffordshire. Journal of Hygiene (Cambridge) 1968;66:649–655. doi: 10.1017/s0022172400028382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hahn G, Koch P.Coxiella burnetii. Monograph on the significance of pathogenic microorganisms in raw milk. Special Issue No. 9405. Brussels, Belgium: IDF Group of Experts AlO/Al1. International Dairy Federation, 1993
- 58.Tissot-Dupont H, Raoult D. Epidemiology of Q fever [in French] Bulletin Épidémiologique Hebdomadaire. 1993;1993:17–18. [Google Scholar]
- 59.Tselentis Y et al. Q fever in the Greek Island of Crete: epidemiologic, clinical, and therapeutic data from 98 cases. Clinical Infectious Diseases. 1995;20:1311–1316. doi: 10.1093/clinids/20.5.1311. [DOI] [PubMed] [Google Scholar]
- 60.Suarez-Estrada J et al. Seroepidemiological survey of Q fever in Leon Province, Spain. European Journal of Epidemiology. 1996;12:245–250. doi: 10.1007/BF00145413. [DOI] [PubMed] [Google Scholar]
- 61.Combiesco D. Pulmonary typhus fever – pulmonary rickettsiosis [in French] Archives Roumaines des Pathologies Expérimentales et de Microbiologie. 1957;16:37–55. [Google Scholar]
- 62.Benson WW, Brock DW, Mather J. Serologic analysis of a penitentiary group using raw milk from a Q fever infected herd. Public Health Report. 1963;78:707–710. [PMC free article] [PubMed] [Google Scholar]
- 63.Durand MP, Limouzin C. A food hygiene problem: a propos the potential risk on human health of cow milk infected by Coxiella burnetti [in French] Bulletin de l'Académie Vétérinaire de France. 1983;56:475–485. [Google Scholar]
- 64.Garner MG et al. A review of Q fever in Australia 1991–1994. Australia and New Zealand Journal of Public Health. 1997;21:722–730. doi: 10.1111/j.1467-842x.1997.tb01787.x. [DOI] [PubMed] [Google Scholar]
- 65.Kermode M et al. An economic evaluation of increased uptake in Q fever vaccination among meat and agricultural industry workers following implementation of the National Fever Management Program. Australia and New Zealand Journal of Public Health. 2003;27:390. doi: 10.1111/j.1467-842x.2003.tb00415.x. [DOI] [PubMed] [Google Scholar]
- 66.La Scola B, Lepidi H, Raoult D. Pathologic changes during acute Q fever: influence of the route of infection and inoculum size in infected guinea pigs. Infection and Immunity. 1997;65:2443–2447. doi: 10.1128/iai.65.6.2443-2447.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.de Alarcon A et al. Q fever: epidemiology, clinical features and prognosis. A study from 1983 to 1999 in the South of Spain. Journal of Infection. 2003;47:110–116. doi: 10.1016/s0163-4453(03)00013-6. [DOI] [PubMed] [Google Scholar]
- 68.Tiggert WD, Benenson AS. Studies on Q fever in man. Transactions of the Association of American Physicians. 1956;69:98–104. [PubMed] [Google Scholar]
- 69.Marrie T et al. Exposure to parturient cats is a risk factor for acquisition of Q fever in Maritime Canada. Journal of Infectious Clinical Diseases. 1988;319:354–356. doi: 10.1093/infdis/158.1.101. [DOI] [PubMed] [Google Scholar]
- 70.Domingo P et al. Acute Q fever in adult patients: report on 63 sporadic cases in an urban area. Clinical Infectious Diseases. 1999;29:874–879. doi: 10.1086/520452. [DOI] [PubMed] [Google Scholar]
- 71.Raoult D et al. Q fever 1985–1998. Clinical and epidemiologic features of 1,383 infections. Medicine. 2000;79:109–123. doi: 10.1097/00005792-200003000-00005. [DOI] [PubMed] [Google Scholar]


