SUMMARY
Outbreaks of enteric disease associated with exposure to live animals on exhibit have occurred with increasing frequency in recent years. Possibly the most important pathogen causing such outbreaks is Escherichia coli O157:H7, because of the serious illness it can cause. Hand hygiene is consistently protective against disease among persons exposed to animals implicated in these outbreaks. Livestock barns have limited hand-washing facilities, therefore a waterless hand-sanitizing gel would be a potentially preventive measure readily available to visitors and animal exhibitors. This study compared the reduction of bacterial counts on hands of animal exhibitors when soap and water was used or when an ethanol-based hand gel was used after animal handling. Participants were youth and adults involved with showing livestock. The sanitation methods were similar in reducing the total bacteria and coliform counts on the hands of the participants (Wilcoxon rank sum test P values 0·12 and 0·69 respectively).
Outbreaks of human enteric disease associated with visiting animal exhibits (state and county fairs, petting zoos, and open farms) are occurring with increasing frequency [1, 2]. Because of the severity of disease it causes, E. coli O157:H7 may be the most important enteric pathogen that has been associated with animal exhibits. E. coli O157:H7 causes haemorrhagic colitis that can progress to hemolytic–uraemic syndrome (HUS) and death, particularly in young children [3]. There have been at least 11 documented outbreaks of E. coli O157:H7 associated with animal exhibits in North America from 1999 to 2005 [1, 2, 4, 5] affecting hundreds of people, among whom dozens developed HUS. Animal exhibit-associated salmonellosis and cryptosporidiosis outbreaks have also been reported [6–8].
E. coli O157:H7 is found ubiquitously among healthy cattle and other ruminants worldwide; cattle herd-level prevalences range from 2 to 100% with a variety of detection methods [9]. Although Salmonella enterica and Cryptosporidium parvum are associated with diarrhoeal disease in ruminants and other animals, they also are often shed by healthy animals. Like E. coli O157:H7, they are ubiquitous among livestock animals [10, 11]. As these pathogens can be spread by direct contact with animals, interventions that block transmission of E. coli O157:H7 will probably have the effect of interrupting the transmission of other enteric pathogens as well.
The protective effect of hand-washing after animal contact and before eating has been a consistent finding in many animal exhibit-associated outbreak investigations [7, 12, 13]. Hand washing with soap and water is the cornerstone of recommendations to prevent transmission of zoonotic pathogens at animal exhibits [14]. In public animal exhibits, however, the low ratio of hand-washing sinks to people often creates situations where soap-and-water washing is inconvenient and, therefore, not done. In two case-control studies of animal exhibit-associated outbreaks, the prevalence of hand washing in controls ranged from 45 to 55% [7, 12]. Even among health-care providers, an educated and motivated group, compliance with hand hygiene recommendations ranged from 4 to 60% before educational interventions, and never reached 100% even after education was implemented [15]. Soap-and-water hand-washing is particularly inconvenient in livestock exhibit barns, because they often lack indoor hand-washing sinks. In addition, the design of mobile hand-washing stations often makes them difficult to use, especially for small children, the highest risk group.
An alternative to soap-and-water hand-washing is the use of alcohol-based hand gels. Studies evaluating hand hygiene in health-care settings have found consistently that waterless alcohol-based preparations are effective at killing and removing hand bacterial flora [15]. It is thought that, because alcohol-based gels do nothing to remove gross contamination, this method is not appropriate when hands are visibly soiled [15, 16]. To our knowledge, alcohol-based hand gels have never been tested as an alternative to soap-and-water washing in the context of animal handling. This report describes a study designed to evaluate the effectiveness of alcohol-based hand gels in the context of handling ruminant animals.
Closed livestock shows are events in which 4H and Future Farmers of America (FFA) youth exhibit animals for show and sale purposes, but are not open to the general public. They take place annually on state and county fairgrounds in the Palouse region of Washington and Idaho states and include cattle, sheep and goat shows. Local 4H and FFA groups agreed to allow the research group to ask for volunteers among the affiliated youth at pre-event meetings. Informational pamphlets describing the study were distributed at these meetings, along with consent forms for parental signatures. The project was approved by the University of Idaho Human Subjects Review Board.
Before each event, a random number list was generated in Microsoft Excel (Redmond, WA, USA) to determine group allocation. This random number list was transferred to a series of small stickers. As participants arrived at the event and before handling their animals, they were each given a numbered sticker and thus allocated to one of two groups, the soap-and-water group or the hand-gel group. Consent forms signed by parents or guardians were required for participation by minors. After each participant was given a numbered sticker, he or she was asked to wash their hands by wetting their hands, lathering for 10 s with an antibacterial soap and then rinsing for 10 s under running water. Hands were then dried with paper towels. This step was included to remove transient bacterial flora from the hands of exhibitors. To measure baseline bacterial counts on hands, a pre-handling sample was taken from the first 31 participants by the method described below. The exhibitors then interacted with their animals as they normally would in preparation for a show. After handling their animals, exhibitors returned to the hand hygiene station and were asked to rub both hands together in a circular motion 20–25 times. This was to reduce potential bias introduced by right- or left-handedness. One hand was then sampled (pre-hygiene sample). Those allocated to the soap-and-water group then washed their hands with soap and running water, lathering for 10–20 s, and rinsed their hands with running water, then dried hands with a paper towel. The other hand was then sampled (post-hygiene sample). Those allocated to the gel group applied 1–3 ml of 62% ethanol-based hand gel and allowed their hands to air-dry for 60 s. Pre- and post-hygiene samples were taken from those in the gel group using the same protocol.
Prior to each event, plastic Ziploc® bags (S. C. Johnson, Racine, WI, USA) containing 100 ml rinse solution were prepared. Rinse solution consisted of Letheen broth (Acumedia Manufacturers, Baltimore, MD, USA) in 0·075 m phosphate buffer (pH 7·9), with 0·1% Triton X-100, 0·5% lecithin, 0·5% sodium thiosulphate, and 1·0% Tween-80 [17]. Rinse solutions were held at 4°C until transportation to the animal exhibit event, and used solutions were kept in a cooler with ice packs until transportation back to the laboratory. To sample hands, each participant was asked to immerse the hand being sampled into the bag with solution and vigorously move their fingers for 60 s. After the final sample was taken, participants were asked to wash their hands with soap-and-water and to towel-dry before leaving the premises.
Serial dilutions of the rinse solution were spread plated onto Violet Red bile agar with 4-methylumbelliferylbeta-d-glucuronide (VRB-MUG) and onto Luria–Bertani (LB) media, and incubated overnight at 37°C. The number of coliforms (pink colonies on VRB), generic E. coli (pink colonies and fluorescent colonies on VRB) and total bacterial counts (total colonies on LB) were enumerated. For each group of participants, a control sample of rinse solution from an unused bag was cultured using the same protocol as for samples.
The reduction in counts between pre- and post-hygiene was calculated by taking the log10 of the bacterial counts and then subtracting post-hygiene values from pre-hygiene values for coliforms and total bacteria. A value of 1 was substituted for a 0 bacterial count for the log transformation. Mean pre- and post-hygiene count reductions were compared between treatment groups using a Wilcoxon rank sum test for independent samples. Data were entered and stored in Excel (Microsoft) and analysed using SAS software (SAS Institute, Cary, NC, USA).
Five animal-exhibit groups participated in the study. Group I consisted of 4H sheep exhibitors who participated in a practice showing at a private farm in eastern Washington. Group II consisted of a 4H team who conducted a tour of farms and handled their animals at their home farms. Group III consisted of 4H and FFA exhibitors at a small county fair in rural Idaho. Group IV was drawn from 4H and FFA exhibitors at a large county fair in central Washington, and group V consisted of adults participating in sheep-judge training in eastern Washington. The two treatment groups were similar with regard to median age, gender, and animal species exhibited. Hands of participants in groups I–III were sampled before they handled their animals. For these 31 participants, the baseline total bacterial counts were similar between treatment groups (Table).
Table.
Characteristics of hand hygiene groups
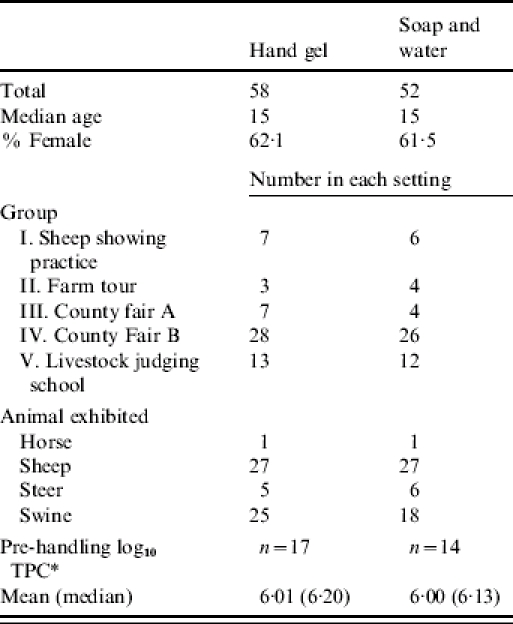
Log10 of total plate counts on hands before handling animals, groups I–III.
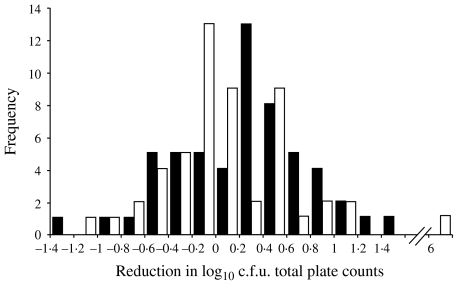
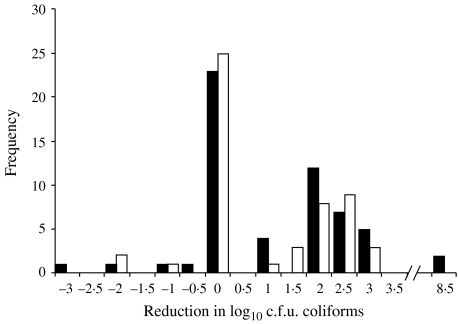
The distribution of bacterial reduction after use of ethanol-based hand gel was similar to that distribution among those who used soap and water. A reduction of less than zero resulted when counts were higher post-hygiene than they were pre-hygiene. For total bacterial counts, the log reduction ranged from −1·4 to 6 logs. The Wilcoxon rank sum two-sided P value for the difference between the two groups was 0·12 (Fig. 1). There was also no significant difference between the reduction in the log of coliform counts between the two groups. Many of the participants had no coliforms detected on their hands either pre- or post-hygiene. The Wilcoxon rank sum two-sided P value for the difference between the two groups was 0·69 (Fig. 2). Among all of the study participants, 14 had E. coli counts on hands after handling their animals (data not shown). Of these 14, seven were in the hand-gel group and seven were in the soap-and-water group. All of those in the gel group had no E. coli detected on their hands post-hygiene. Three of the soap-and-water group had low counts of E. coli detected on their hands post-hygiene (Fisher's exact 2-tailed P value=0·19). In 44 cases (26 among the soap-and-water group and 18 among the hand-gel group, χ2P=0·09), the number of total bacteria recovered after the hygiene activity was higher than the number of bacteria recovered prior to the activity. In seven cases (three among the soap-and-water group and four among the hand-gel group, Fisher's exact 2-tailed P value=1·0), this phenomenon of increased numbers was also observed for coliform counts. None of the control samples yielded colonies on either LB or VRB-MUG plates.
Fig. 1.
Distribution of the reduction in log10 total plate counts on hands after use of ethanol-based hand gel (■) and soap and water (□). Wilcoxon rank sum test two-sided P=0·12.
Fig. 2.
Distribution of the reduction in log10 total coliforms on hands after use of ethanol-based hand gel (■) and soap and water (□). Wilcoxon rank sum test two-sided P=0·69.
This study found no detectable difference between the effectiveness of ethanol-based hand gel and soap and water in reducing microbial counts on the hands of livestock exhibitors. This finding is encouraging in the context of recent E. coli O157:H7 disease outbreaks at agricultural fair petting zoos in North Carolina [4] and Florida [5]. Livestock barns at state and county agricultural fairs rarely have adequate, if any, hand-wash sinks or stations inside the facility. Animal exhibitors often must spend hours inside livestock barns where their show animals are housed. In such situations, or when hand-wash stations are not adequate for the volume of visiting public, the use of a portable hand sanitizer is a potential preventive step.
This is the first study of its kind to the authors' knowledge and represents a preliminary step for future research efforts. The solution to the problem of disease transmission in these kinds of settings must be innovative and has to accommodate the variability of livestock settings and county resources. Agricultural fairs and petting zoos can range from a small former commercial dairy to a large state fair barn housing hundreds of animals, therefore, recommendations aimed at preventing disease transmission that are appropriate for all of these settings are difficult to establish.
Two major drawbacks of the current study were the limited statistical power and the lack of a blind design in the study. In many cases, the total number of bacteria recovered after the hygiene activity was higher than the number of bacteria recovered prior to the activity, regardless of the hygiene method. This phenomenon may have been due to loosening of normal microbial flora from hands during the hygiene activity, or because rubbing the hands together was inadequate to prevent potential bias from handedness. The choice of the pre-hygiene sampled hand was left up to the participant and this self-selection could have introduced bias in either direction. A similar observation of increased numbers post-hygiene was uncommon for Gram-negative coliform counts, and may be attributable to chance, because coliforms were present in low numbers in the sample solution. The results reported here do not have a bearing on Cryptosporidium which is a protozoan pathogen. Future research in this area should include larger numbers of participants, a study design in which investigators are blinded to the group allocation of each sample, and evaluation of a variety of preparations that could also be effective against protozoan pathogens. Even if their efficacy becomes established, hand sanitizers can only be part of the solution to the problem of zoonotic disease transmission. Other strategies including the placement of barns and food concessions, signposting, optimal animal housing, appropriate barriers to animal pens and adequate hand-wash sinks are critical [14].
ACKNOWLEDGEMENTS
This work was supported, in part, by the Idaho Agriculture Experiment Station, the National Research Initiative of the USDA Cooperative State Research, Education and Extension Service, grant number 04-04562, Public Health Service grants NO1-HD-0-3309, U54-AI-57141, P20-RR16454, and P20-RR15587 from the National Institutes of Health, and by grants from the United Dairymen of Idaho and the Idaho Beef Council. The authors acknowledge 4H groups in Latah County, Idaho and in Whitman County, Washington for their interest and participation in this project. In particular, we acknowledge the contributions of Lonie and Marilyn Austin, Jan Busboom, and Mari Etta Leitch. For invaluable technical support, we acknowledge Hannah Knecht, Heidi Hobbs and Richard Knight, and for expert advice we thank Daniel H. Rice.
DECLARATION OF INTEREST
None.
REFERENCES
- 1.Bender JB, Shulman SA. Reports of zoonotic disease outbreaks associated with animal exhibits and availability of recommendations for preventing zoonotic disease transmission from animals to people in such settings. Journal of the American Veterinary Medical Association. 2004;224:1105–1109. doi: 10.2460/javma.2004.224.1105. [DOI] [PubMed] [Google Scholar]
- 2.LeJeune JT, Davis MA. Outbreaks of zoonotic enteric disease associated with animal exhibits. Journal of the American Veterinary Medical Association. 2004;224:1440–1445. doi: 10.2460/javma.2004.224.1440. [DOI] [PubMed] [Google Scholar]
- 3.Besser RE, Griffin PM, Slutsker L. Escherichia coli O157:H7 gastroenteritis and the hemolytic uremic syndrome: an emerging infectious disease. Annual Review of Medicine. 1999;50:355–367. doi: 10.1146/annurev.med.50.1.355. [DOI] [PubMed] [Google Scholar]
- 4.Goode B, O'Reilly C. Outbreak of Shiga toxin producing E. coli STEC. infections associated with a petting zoo at the North Carolina State Fair – Raleigh, North Carolina, November 2004. Final Report: General Communicable Disease Control Branch, Division of Public Health, North Carolina Department of Health and Human Services; 29 June 2005.
- 5.ProMED-mail ProMED-mail; 2005. . Hemolytic uremic syndrome, petting zoo – USA FL. ; 20050404.0964. [Google Scholar]
- 6.Seattle – King County Department of Public Health. Salmonellosis linked to animal exhibit. Epi-Log. 1991;4:1. [Google Scholar]
- 7.Friedman CR et al. An outbreak of salmonellosis among children attending a reptile exhibit at a zoo. Journal of Pediatrics. 1998;132:802–807. doi: 10.1016/s0022-3476(98)70307-5. [DOI] [PubMed] [Google Scholar]
- 8.Smith KE et al. Outbreaks of enteric infections caused by multiple pathogens associated with calves at a farm day camp. Pediatric Infectious Diseases Journal. 2004;23:1098–1104. [PubMed] [Google Scholar]
- 9.Meyer-Broseta S et al. Review of epidemiological surveys on the prevalence of contamination of healthy cattle with Escherichia coli serogroup O157:H7. International Journal of Hygiene and Environmental Health. 2001;203:347–361. doi: 10.1078/1438-4639-4410041. [DOI] [PubMed] [Google Scholar]
- 10.Atwill ER et al. Age, geographic, and temporal distribution of fecal shedding of Cryptosporidium parvum oocysts in cow-calf herds. American Journal of Veterinary Research. 1999;60:420–425. [PubMed] [Google Scholar]
- 11.USDA Salmonella and Campylobacter on US dairy operations, 2003
- 12.Crump JA et al. An outbreak of Escherichia coli O157:H7 infections among visitors to a dairy farm. New England Journal of Medicine. 2002;347:555–560. doi: 10.1056/NEJMoa020524. [DOI] [PubMed] [Google Scholar]
- 13.Evans M, Gardner D. Cryptosporidiosis outbreak associated with an educational farm holiday. Communicable Disease Report. CDR Review. 1996;6:R50–R51. [PubMed] [Google Scholar]
- 14.CDC. Compendium of measures to prevent disease associated with animals in public settings, 2005: National Association of State Public Health Veterinarians, Inc. Morbidity and Mortality Weekly Report. 2005;54:1–13. [PubMed] [Google Scholar]
- 15.CDC. Guideline for hand hygiene in health-care settings: recommendations of the Healthcare Infection Control Practices Advisory Committee and the HICPAC/SHEA/APIC/IDSA Task Force. Morbidity and Mortality Weekly Report. 2002;51:1–45. [PubMed] [Google Scholar]
- 16.Widmer AF. Replace hand washing with use of a waterless alcohol hand rub. Clinical Infectious Diseases. 2000;31:136–143. doi: 10.1086/313888. [DOI] [PubMed] [Google Scholar]
- 17.Larson EL, Strom MS, Evans CA. Analysis of three variables in sampling solutions used to assay bacteria of hands: type of solution, use of antiseptic neutralizers, and solution temperature. Journal of Clinical Microbiology. 1980;12:355–360. doi: 10.1128/jcm.12.3.355-360.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]