SUMMARY
The aims were to (1) investigate the aetiology of probable meningococcal disease, where a clinical diagnosis is made in the absence of laboratory data, and (2) evaluate the impact of the Men C vaccination programme in England and Wales. Multiple linear regression analyses were carried out using data reported to Enhanced Surveillance of Meningococcal Disease (ESMD) and laboratory reports of isolates of organisms causing symptoms that mimic meningococcal disease. Confirmed meningococcal disease appeared to be a significant predictor of probable disease. Thus, an additional reduction in meningococcal disease attributable to the serogroup C vaccination campaign was evident in probable disease over and above that observed in confirmed cases alone. Enteroviruses were a significant contributor to cases of probable meningitis and influenza appeared to be a significant contributor to probable cases of septicaemia. This analysis confirms the success seen following the Men C vaccination campaign and gives an indication of the aetiologies of other causes of probable meningitis and septicaemia reported to ESMD.
INTRODUCTION
Meningococcal disease, including meningitis and septicaemia, is caused by the organism Neisseria meningitidis. The majority of meningococcal infections occur in infants <5 years of age, with a peak incidence in those <1 year [1]. There is a secondary peak in incidence in young adults aged between 15 and 19 years of age. Most infections in the United Kingdom are due to serogroups B and C. The case fatality is ∼10%, with more deaths occurring from septicaemia than meningitis [1].
A national vaccination campaign for serogroup C meningococcal disease began in the United Kingdom on 29 November 1999, when a new meningococcal conjugate serogroup C vaccine (Men C) was introduced into the routine childhood schedule alongside a catch-up programme in older children [2]. A phased introduction was necessary due to limited supplies of the vaccine, initially targeting high-risk groups (infants, and teenagers aged 15–17 years) [3].
The positive impact of the campaign was reflected in a decline in isolates and samples of serogroup C, but not serogroup B, meningococcal disease referred to the Health Protection Agency Meningococcal Reference Unit (MRU) in vaccinated age groups [4]. Although improved methods for laboratory confirmation, in particular PCR [5], are available, it is not possible to confirm all cases of meningococcal disease. Enhanced Surveillance of Meningococcal Disease (ESMD) was introduced to improve estimates of the burden of meningococcal disease prior to implementation of the Men C vaccine and to monitor meningococcal disease post-implementation. In the ESMD programme, information on clinically diagnosed meningococcal disease is reconciled with laboratory data from the MRU. When a case has not been confirmed by a laboratory, it is classified as a probable case based on clinical case definitions. Unlike confirmed cases, probable disease has shown no obvious trend among the age groups vaccinated with Men C, suggesting that some probable cases may be caused by other pathogens. Many different organisms can cause symptoms that may be mistaken for meningococcal disease, particularly septicaemia.
In order to calculate the impact of vaccination on probable meningococcal disease, the aetiology of these probable cases must first be determined. ESMD was used to examine the relationship between probable and confirmed cases and to estimate the possible contribution of viral and other causes of meningitis and septicaemia. As meningococcal disease shows a clear seasonality, any seasonal pattern in probable disease should correspond to that in confirmed disease. Seasonal patterns in cases reported to ESMD between January 1999 and June 2003 were, therefore, investigated using statistical models and laboratory data. The objectives were to (1) determine the aetiology of probable meningococcal disease, (2) evaluate the impact of the Men C vaccine on these probable cases, and (3) compare the aetiology of probable cases with a diagnosis of meningitis to those with a diagnosis of septicaemia.
METHODS
Data sources
ESMD
All cases of meningococcal disease from England and Wales reported through ESMD between 1 January 1999 and 30 June 2003 were included in this analysis. Probable cases of meningococcal disease are defined as:
meningitis, septicaemia or other invasive disease in the absence of laboratory confirmation where N. meningitidis is thought to be the most likely diagnosis by the clinician managing the case and/or the Consultant in Communicable Disease Control [6].
Cases of laboratory-confirmed meningococcal disease were classified into serogroup B, serogroup C or ‘other’ (ungrouped or attributed to serogroups W135, Y, 29-E, X or Z). Additionally, cases of confirmed and probable meningococcal disease were grouped by diagnosis (meningitis vs. septicaemia) for further analysis. Cases were grouped into the following age categories; <1, 1–4, 5–14, 15–19 and ⩾20 years. Four-week totals were calculated.
Laboratory reports
Many different organisms can cause symptoms that may be mistaken for meningococcal disease, particularly septicaemia. The possible organisms for inclusion in the model were identified by enhanced surveillance in 1998, where alternative diagnoses were recorded for cases initially suspected to be attributable to meningococcal disease [3]. These include pneumococcal infection, streptococcal (A or B) infection, Haemophilus influenzae, enterovirus (type 70, 71 or untyped), coxsackie B virus, echovirus, influenza (A, B or ungrouped), RSV, herpes simplex virus, varicella zoster virus, parvovirus, and mumps virus. In order to qualify for consideration in the regression models, laboratory reports for these pathogens had to exhibit a seasonal pattern and be reported frequently enough that their seasonality was apparent.
Four-week totals of laboratory reports of isolates of the organisms identified above for the period 1 January 1999 to 30 June 2003 were obtained from confirmed infections reported to the Health Protection Agency Centre for Infections from a network of over 250 laboratories in England and Wales.
Evaluating impact of Men C vaccine programme
Multiple linear regression analyses, carried out in Microsoft Excel, were used to assess the contribution of meningococci and other organisms to probable cases of meningococcal disease (including septicaemia, meningitis and other invasive diseases combined) and subsequently to estimate the number of cases of probable meningococcal disease attributable to serogroup C N. meningitidis. This method relies on the seasonal variation of meningococcal disease and other infections included in the model, and how the variation is reflected in the number of four-weekly total reports. The methods have been used in a similar way to investigate hospital admissions due to rotavirus [7], and the contribution of RSV to bronchiolitis and pneumonia-associated hospitalizations [8]. The model associates ESMD four-weekly totals of confirmed meningococcal disease, as well as other organisms that can cause symptoms that may be mistaken for meningococcal disease, with probable cases. The goodness-of-fit of the model is denoted by R2.
The formula for estimated probable cases Yj in the 4-week period j:
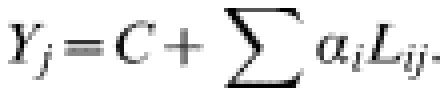 |
Where Lij is the number of laboratory or ESMD reports of type i in the 4-week period j and αi is the number of probable cases of meningococcal disease associated with each report of type i. C is a constant representing the background number of probable cases in each 4-week period associated with other infectious or non-infectious causes of meningitis, septicaemia or other clinical illness which may be mistaken for either of these syndromes, where the temporal trend is not strong enough to be individually significant. The values of α were estimated by least squares regression. An organism was only retained in the model if α was found to be significantly associated with probable disease (P<0·05). This method assumes that a confirmed case of each organism is associated with a constant number of probable cases of meningococcal disease.
The statistical models for each age group generated a value for the number of probable cases associated with a single confirmed case of serogroup C (α). These values were applied to six-monthly totals of confirmed serogroup C cases to estimate the total number of cases of probable meningococcal disease reported in ESMD that were due to serogroup C meningococcal infection. Similarly, six-monthly rates for probable serogroup C disease were estimated per 100 000 of population based on age-specific 2001 population estimates for England and Wales.
Further investigating the aetiology of probable cases by diagnosis
To further investigate the aetiology of probable meningococcal disease by diagnoses, ESMD data were broken down into probable septicaemia and confirmed serogroup-specific septicaemia cases and probable meningitis and confirmed serogroup-specific meningitis cases. Multiple linear regression analyses were carried out, as described above, for the diagnoses of meningitis and septicaemia separately. Laboratory data for the identical organisms mentioned previously were included in the models.
RESULTS
Enhanced surveillance of meningococcal disease
Between 1 January 1999 and 30 June 2003, 17 440 cases of meningococcal disease were identified by ESMD. Of these, 7471 (43%) were probable cases rather than laboratory confirmed. Of the 9969 confirmed cases, 67% were serogroup B, 22% were serogroup C and 11% were ungrouped or another serogroup (classified as ‘other’; Table 1). There were more cases of septicaemia than meningitis overall, serogroup C was more common in cases of septicaemia and in older age groups, and amongst probable cases a lower proportion had a diagnosis of septicaemia (Table 1).
Table 1.
Breakdown of cases reported to Enhanced Surveillance of Meningococcal Disease (ESMD) by age group, serogroup and diagnosis, January 1999 to end June 2003*
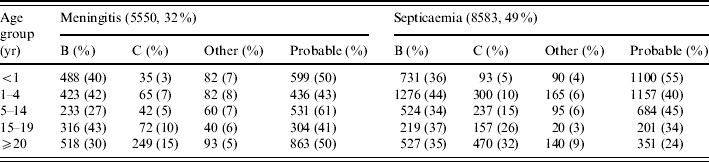
Cases reported to ESMD with a diagnosis of both meningitis and septicaemia, other invasive disease excluding meningitis and septicaemia or where the diagnosis was not known have been excluded from this table, as have cases for which the age group was not known.
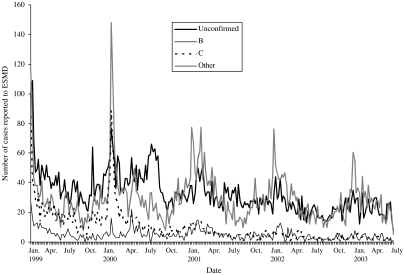
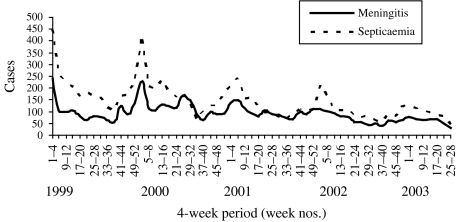
The seasonal pattern of probable meningococcal disease is less striking than confirmed meningococcal disease, and seasonality varied among age groups and by disease presentation (Fig. 1). Although there is a peak in both meningitis and septicaemia in the winter, the peaks are more marked for septicaemia (Fig. 2).
Fig. 1.
Cases of confirmed and probable meningococcal disease as reported to ESMD by week (week 1, 1999 to week 27, 2003).
Fig. 2.
Seasonal patterns of meningitis and septicaemia (confirmed and probable) reported to ESMD, January 1999 to June 2003.
Laboratory reports
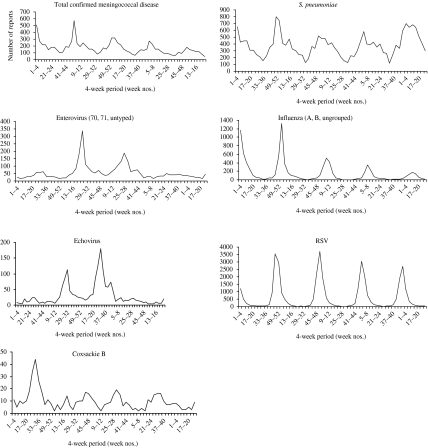
Some infections display regular annual winter peaks (e.g. N. meningitidis, S. pneumoniae, RSV) and others are less regular and have peaks in the summer (e.g. enteroviruses; Fig. 3). Enteroviruses, a major cause of viral meningitis, have a summer peak in reports seen each year, and a particularly large outbreak occurred in 2000 [9].
Fig. 3.
Seasonal patterns (weeks 14–39=summer half year; week 40 to week 13 the following year=winter half year) of confirmed meningococcal disease, S. pneumoniae, enterovirus, influenza, echovirus, RSV, and coxsackie B virus.
Evaluating the impact of the Men C vaccine programme
In assessing the contribution of meningococci and other organisms to probable cases of meningococcal disease, the model appeared to fit best in children aged 1–4 years (R2=0·77) and worst in teenagers aged 15–19 years (R2=0·48, Table 2). Serogroup C meningococcal disease was a significant contributor in all age groups except for the ⩾20 years group. In these age groups ∼20% of probable cases were attributed to serogroup C meningococcal disease. Influenza was found to be significantly associated with probable meningococcal disease in the 1–4, 5–14 and 15–19 years age groups, and enterovirus was significantly associated with probable meningococcal disease in infants, children aged 5–14 years, and ⩾20-year-olds. Serogroup B and other serogroups were also important contributors in the ⩾20 years age group. The values of α, the number of probable cases of serogroup C meningococcal disease associated with a confirmed case, showed that the estimated increase in burden of meningococcal serogroup C is greatest in the younger age groups, with a 228% increase in cases in the <1 year age group (Table 2). This falls with increasing age to 46% in the 15–19 years age group.
Table 2.
Results of multiple linear regression by age group: fit of the model (R2), significant contributors to probable meningococcal disease, and number of probable cases associated with a single confirmed case of serogroup C (α)
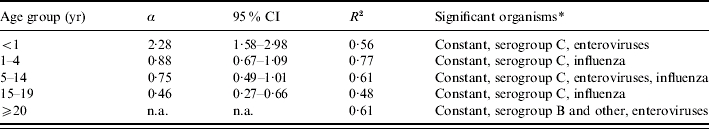
n.a., Not available as serogroup C not significant contributor of probable disease in this group.
All organisms identified as causing symptoms that mimic meningococcal disease were fitted to the model but only retained if found to be significant (P<0·05).
The additional number of probable cases that were estimated to be due to serogroup C meningococcal disease was calculated for each age group by a 6-month period between week 1 (1999) and week 26 (2003). Overall, an additional 1170 cases of probable disease were estimated to be attributable to serogroup C meningococcal infection (Table 3). An estimated 1009 cases of serogroup C meningococcal disease (both confirmed and probable) occurred in the first 6 months of 1999 compared to only 79 in the same period of 2003. This represents a reduction of ∼92%, higher than that estimated from surveillance of confirmed cases alone (just below 90%). The greatest reduction in the rate of serogroup C meningococcal disease (confirmed and probable) before and after the vaccination campaign is seen in infants <1 year old (Table 4).
Table 3.
Total number confirmed and estimated number probable cases attributed to serogroup C meningococcal infection by age group (week 1, 1999 to week 26, 2003)
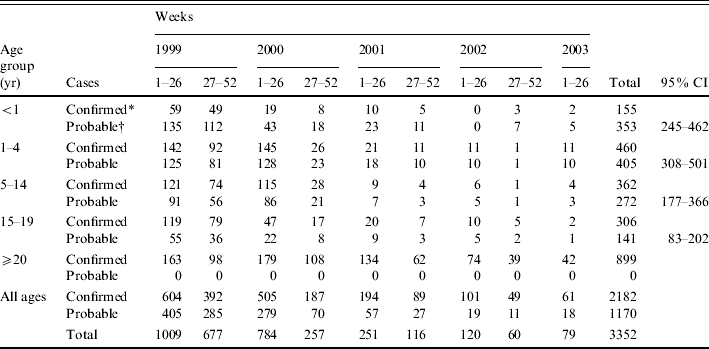
Observed number of confirmed cases.
Estimated number of probable cases.
Table 4.
Rate of serogroup C meningococcal disease (confirmed and probable) by age group (weeks 1–26, 1999 and weeks 1–26, 2003)
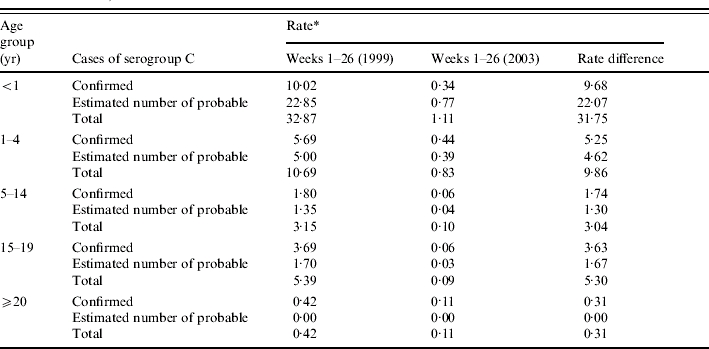
Six-monthly rate is estimated per 100 000 population based on age-specific 2001 population estimates for England and Wales.
Further investigating the aetiology of probable cases by diagnosis
Meningitis
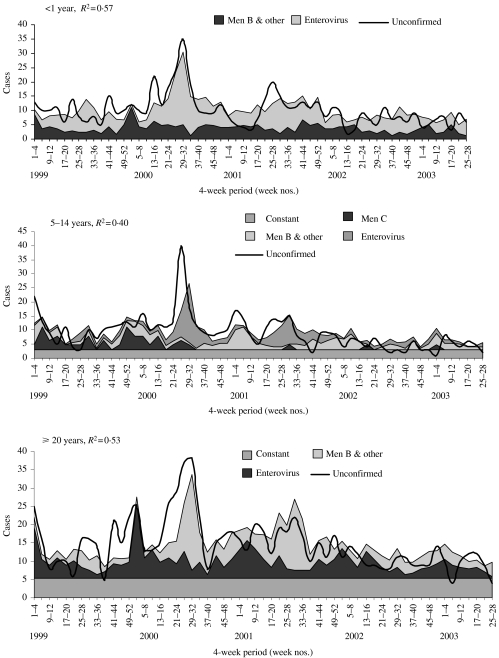
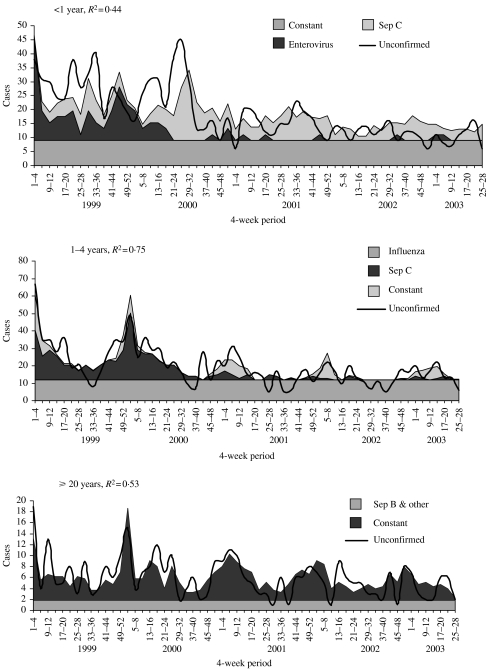
For meningitis, the highest R2 value was seen in the <1 year age group (R2=0·57). This was followed by the ⩾20 and 5–14 years groups, with an R2 of 0·53 and 0·40 respectively (Fig. 4). The model fits particularly poorly in the 15–19 and 1–4 years age groups. The contribution of meningococcal infection to probable meningitis varied by age. In the <1, 5–14 and ⩾20 years age groups, between 26% and 38% of all probable cases were attributed to meningococcal disease caused by serogroup B and other serogroups. In the 1–4, 5–14 and 15–19 years age groups, between 10% and 21% of all probable cases were attributed to meningococcal serogroup C. Enterovirus infection was significantly associated with probable disease in all age groups apart from 1–4 and 15–19 years. No other organism was found to be significantly associated with probable disease. In children <1 year old, 62% of probable cases were attributed to enterovirus infection. In all ages apart from those <1 year old, a constant was retained and accounted for between 34% and 90% of probable cases.
Fig. 4.
Multiple regression models, fitted to probable cases of meningococcal meningitis, for age groups where model had ‘good fit’ (R2>0·40).
Septicaemia
For septicaemia, the highest R2 value was seen in the 1–4 years age group (R2=0·75). This was followed by the ⩾20, 5–14, 15–19 and <1 years age groups, with R2 values ranging between 0·44 and 0·53 (Fig. 5). The contribution of meningococcal infection to probable cases of septicaemia varied. Between 18% and 34% of all probable cases of septicaemia were attributed to meningococcal serogroup C disease in all age groups apart from the ⩾20 years age group. In the latter, meningococcal disease caused by serogroup B and other serogroups explained 70% of probable cases. Enteroviruses were again significantly associated with probable meningococcal disease in those aged <1 year. Influenza also appeared to be associated with probable septicaemia in the 1–4 and 5–14 years age groups. In all ages a constant was retained, and accounted for between 30 and 66% of probable cases.
Fig. 5.
Multiple regression models fitted to probable cases of meningococcal septicaemia, by age group.
DISCUSSION
Confirmed meningococcal disease was significantly associated with probable meningitis and septicaemia for all age groups confirming that N. meningitidis contributes to probable cases reported in the ESMD. This justifies the inclusion of probable cases in the enhanced surveillance conducted in England and Wales.
Over and above the observed reduction in confirmed cases, an additional reduction in meningococcal disease attributable to the serogroup C vaccination campaign can be seen in probable cases. This reduction is most striking in infants, in whom routine coverage for Men C was almost 90% by the end of June 2003 [10]. These children, along with teenagers aged 15–17 years, were the first to receive the vaccine at the start of the campaign, and the reduction in confirmed and probable disease is consistent with high levels of coverage achieved early in the vaccination campaign [4]. As for confirmed disease, serogroup C appeared to be a more important contributor to probable septicaemia than probable meningitis. This explains the observation of a substantial rise in notified meningococcal septicaemia between 1989 and 1995, accompanying the increased proportion of serogroup C amongst confirmed cases [11]. As septicaemia is associated with a higher case-fatality ratio than meningitis [1], this suggests that the impact of the vaccination campaign on deaths from meningococcal infection is likely to be dramatic.
The aetiology of probable meningococcal disease and the suitability of the model in attributing causes to these cases vary between the different age groups and by diagnosis. The goodness of fit of the model depends upon the organisms included. The model is limited to those organisms for which surveillance data are available and excludes as yet unrecognized organisms, organisms for which there are no diagnostic tests or those not routinely reported, for example Epstein–Barr virus. In addition, organisms that do not have a clear seasonality will not be detected by this modelling.
In addition to confirmed N. meningitidis, enteroviruses and influenza were significant contributors to probable cases reported in the ESMD. Enterovirus appeared to be a more important contributor to probable meningitis, while influenza was more important for septicaemia.
Even though the fit (R2 range 0·40–0·57) was not great, the models strongly suggest that enterovirus was a major contributor to the peak in cases of probable meningitis seen in 2000 for numerous age groups. This was particularly true for the <1 year group, where enterovirus contributed to over 60% of probable cases. The peak in viral meningitis notifications in the summer of 2000 was attributed to an increase in echovirus type 13 [9]. Suspected meningococcal meningitis, therefore, should always be investigated for enterovirus. Isolation of enterovirus from the CSF is not always possible, but viral stool culture and enterovirus RNA detection can be used [12, 13]. Improving the laboratory diagnosis of such cases could lead to cost savings for NHS care [14].
Influenza appeared to be a significant contributor to probable cases of septicaemia in numerous age groups. The symptoms of influenza are non-specific and can mimic the symptoms of septicaemia especially in young infants. Influenza can also predispose to meningococcal disease and may be responsible for a small excess number of cases [15]. Influenza is also under-recognized in other paediatric infections [8] and, therefore, clinicians should be more aware and consider investigation for influenza more often.
In addition to demonstrating that ESMD provides additional value over routine surveillance of confirmed infections, this study has identified areas for improvement. Ideally, cases of probable and confirmed disease reported to ESMD should contain information on positive and negative results for laboratory investigations for meningococcal and other infections. This would result in more reliable data on the total incidence of meningococcal disease, including cases that would otherwise be missed.
ACKNOWLEDGEMENTS
We thank Nigel Gay, Caroline Trotter, Usha Gungabissoon, Ian Fryatt and Nick Andrews for their technical help. We also thank all the contributors of ESMD and laboratory data and the Meningococcal Reference Unit in Manchester.
DECLARATION OF INTEREST
None.
REFERENCES
- 1.Health Protection Agency http://www.hpa.org.uk/infections/topics_az/meningo/backgrd.htm. http://www.hpa.org.uk/infections/topics_az/meningo/backgrd.htm . Background information – Meningitis ( ). Accessed 29 September 2005.
- 2.Chief Medical Officer, Chief Nursing Officer, Chief Pharmaceutical Officer London: Department of Health; 1999. . Introduction of immunisation against group C meningococcal infection (PL/CMO/99/2, PL/CNO/99/4, PL/CHO/99/1), [Google Scholar]
- 3.Communicable Disease Surveillance Centre. Vaccination programme for group C meningococcal infection is launched. Communicable Disease Report Weekly. 1999;9:261. [PubMed] [Google Scholar]
- 4.Trotter CL, Ramsay ME, Kaczmarski EB. Meningococcal serogroup C conjugate vaccination in England and Wales: coverage and initial impact of the campaign. Communicable Disease and Public Health. 2002;5:220–225. [PubMed] [Google Scholar]
- 5.Kaczmarski EB et al. Creating a national service for the diagnosis of meningococcal disease by polymerase chain reaction. Communicable Disease and Public Health. 1998;1:54–56. [PubMed] [Google Scholar]
- 6.Davison KL et al. Enhanced surveillance scheme for suspected meningococcal disease in five regional health authorities in England: 1998. Communicable Disease and Public Health. 2002;5:205–212. [PubMed] [Google Scholar]
- 7.Ryan MJ et al. Hospital admissions attributable to rotavirus infection in England and Wales. Journal of Infectious Disease. 1996;174:S12–S18. doi: 10.1093/infdis/174.supplement_1.s12. [DOI] [PubMed] [Google Scholar]
- 8.Muller-Pebody B et al. Contribution of RSV to bronchiolitis and pneumonia-associated hospitalizations in English children. Epidemiology and Infection. 2002;129:99–106. doi: 10.1017/s095026880200729x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Anon. Viral meningitis associated with increase in echovirus type 13. Communicable Disease Report Weekly. 2000;10:277–280. [PubMed] [Google Scholar]
- 10.HPA . COVER programme: April to June 2003. Communicable Disease Report Weekly [serial online] 2003 (accessed 15 December) 13http://www.hpa.org.uk/cdr/archives/2003/cdr3903.pdf
- 11.Ramsay M et al. Changing patterns of case ascertainment and trends in meningococcal disease in England and Wales. Communicable Disease Report Review. 1997;7:49–54. [PubMed] [Google Scholar]
- 12.Corless CE et al. Development and evaluation of a ‘real-time’ RT-PCR for the detection of enterovirus and parechovirus RNA in CSF and throat swab samples. Journal of Medical Virology. 2002;67:555–562. doi: 10.1002/jmv.10138. [DOI] [PubMed] [Google Scholar]
- 13.Public Health Laboratory Service Meningococcus Forum. Endorsed by the Public Health Laboratory Service, Public Health Medicine Environmental Group and Scottish Centre for Infection and Environmental Health. Guidelines for public health management of meningococcal disease in the UK. Communicable Disease and Public Health. 2002;5:187–204. [PubMed] [Google Scholar]
- 14.Marshall GS et al. Potential cost savings through rapid diagnosis of enteroviral meningitis. Pediatric Infectious Disease Journal. 1997;16:1086–1087. doi: 10.1097/00006454-199711000-00015. [DOI] [PubMed] [Google Scholar]
- 15.Jensen ES et al. A 20-year ecological study of the temporal association between influenza and meningococcal disease. European Journal of Epidemiology. 2004;19:181–187. doi: 10.1023/b:ejep.0000017659.80903.5f. [DOI] [PubMed] [Google Scholar]