SUMMARY
During recent years a pandemic clone of Vibrio parahaemolyticus has emerged. Isolates of this clone are distributed among several serotypes, but are genotypically related. In the present study, a phenotyping method (biochemical fingerprinting) was used to characterize pandemic and non-pandemic isolates belonging to V. parahaemolyticus. It was found that the pandemic isolates showed a high level of phenotypic homogeneity and a majority of the pandemic isolates belonged to the same biochemical phenotype, whereas non-pandemic V. parahemolyticus isolates were more heterogeneous. In conclusion, biochemical fingerprinting of V. parahaemolyticus can be used as a first screening method to differentiate between pandemic and non-pandemic isolates of V. parahaemolyticus.
Vibrio parahaemolyticus, a halophilic Gram-negative marine bacterium belonging to the Vibrionaceae family has attracted much attention as the causative agent of human gastroenteritis in the recent past. V. parahaemolyticus is associated with foodborne disease in Japan and throughout Asia since 1950 [1], and is the most frequently isolated Vibrio species associated with gastroenteritis in the United States [2]. The incidence of V. parahaemolyticus infection has increased around the world since 1996, including several large outbreaks in the United States, Southeast Asia, Canada and Mexico [2–5]. Clinical manifestations of V. parahaemolyticus infections include diarrhoea, abdominal cramps, nausea, vomiting, headache, fever and chills, with an incubation period ranging from 4 to 96 h [6]. Human V. parahaemolyticus gastroenteritis is often due to consumption of seafood, including raw and improperly cooked shellfish [7–9]. V. parahaemolyticusis widely disseminated in marine and estuarine environments [9, 10]. Most clinical strains of V. parahaemolyticus produce known virulence factors such as the thermostable direct haemolysin (TDH) encoded by the tdh genes [11], the TDH-related haemolysin (TRH) encoded by the trh genes, and epidemiological evidence supports the view that V. parahaemolyticus strains carrying the tdh and/or the trh gene are virulent [12, 13].
Serotyping is applied for epidemiological investigations of V. parahaemolyticus and more than 75 combinations of O and K serotypes have been described [14]. However, recent studies indicates that a few specific serotypes are more often associated with gastroenteritis [15]. Serotype O4:K12 has been associated with foodborne outbreaks in the United States and Mexico [16], and serotype O3:K6 was recognized as the dominant cause of gastroenteritis in Calcutta, India in 1996 [17]. Since 1996, serotype O3:K6 strains have been responsible for pandemic spread into Southeast Asia, Japan and the United States through travellers and/or ships' ballast water [5, 18]. Furthermore, molecular typing has shown that this pandemic O3:K6 clonal group is distinguishable from O3:K6 strains isolated before 1996 and from other serotypes [17]. However, molecular and epidemiological studies indicate that the recently emerged serotypes O4:K68 and O1:KUT isolated from India and Bangladesh are clonally related to O3:K6 and also possess pandemic potential [19]. Thus, although those serotypes have different antigenic properties belonging to different serotypes, they seem to form one clonal group, recognized as the ‘pandemic group’. Molecular typing methods such as PFGE, RFLP, AP–PCR, ribotyping, sequence determination of tdh, toxR, ORF8 PCR have been applied to discriminate and to find possible clues to the origin of the pandemic group of V. parahaemolyticus [20–22].
Previous studies on the pandemic strains of V. parahaemolyticus have mostly relied on serotyping and molecular typing techniques to discriminate between pandemic and non-pandemic strains. Identification and characterization of isolates below the species level is important in epidemiological studies, e.g. for tracing the source of infections and for identification of epidemic clones. Molecular typing methods which are highly discriminatory are often used for these purposes, but such methods are expensive for handling large numbers of isolates, especially for resource-poor countries. Biochemical fingerprinting using the PhenePlate (PhP) system is a phenotyping system based on kinetic measurements of the fermentation of selected reagents. It has previously been successfully used for identification of virulent clones of Aeromonas spp. [23, 24], Vibrio anguillarum [25] and Escherichia coli [26] in various ecological and epidemiological studies [27, 28]. In this study, we used the PhP biochemical fingerprinting system to understand the differences in phenotypic traits between the pandemic and non-pandemic strains of V. parahaemolyticus.
A total of 190 V. parahaemolyticus isolates including 161 collected from a diarrhoeal surveillance study performed during 1994–2000 at the National Institute of Cholera and Enteric Diseases (NICED), Calcutta, India, and 29 isolates from an international collection of Southeast Asian travellers' diarrhoeal strains were studied. All isolates were confirmed as being V. parahaemolyticus by standard culture and biochemical tests and serotyped by the commercially available V. parahaemolyticus serotyping kits (Denka Seiken Ltd, Tokyo, Japan). All strains had previously been examined for the presence of toxR, tdh and trh genes by PCR assay [29, 30]. Likewise, data on group-specific PCR (GS-PCR) and PCR for open reading frame ORF8 were available from previous investigations [19, 31].
Biochemical fingerprinting of the isolates were performed using the PhP system (PhPlate Microplate Techniques, Stockholm, Sweden) according to the manufacturer's instructions [32]. PhP-RV (a rapid screening method using 11 dehydrated reagents) was initially used for typing 161 isolates, and PhP-48 (using 48 reagents) was used to further discriminate among 58 isolates. According to the data obtained from typing with the PhP-RV system, the 161 isolates were distributed into eight PhP types (data not shown). Among the isolates, 65% clustered into PhP type 1. Within this type, 46% of the isolates belonged to serotype O3:K6, 12% to O4:K68, 8% to O1:K25, 9% to O1:KUT and 25% to different serotypes. Serotype O3:K6 was distributed among seven different PhP types. Thus, the PhP-RV typing system did not allow consistent discrimination of the pandemic group strains from others.
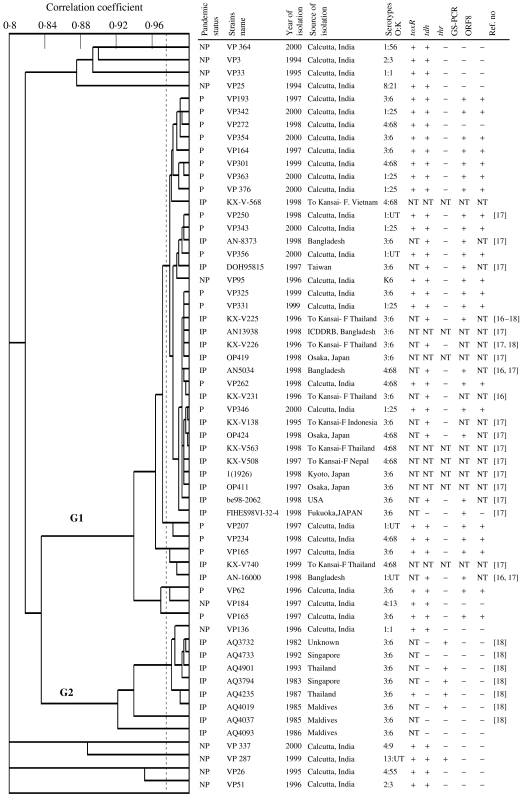
A total of 58 isolates were further typed using the more discriminatory PhP-48 system. Thirty-one of these were randomly selected isolates from NICED Calcutta patients [11 non-pandemic and 20 pandemic strains (seven of O3:K6, six O1:K25, four O4:K68, and three O1:KUT Calcutta patients)] and the other 27 were from Southeast Asia (19 isolated in 1996 or later, and eight isolated before 1996). UPGMA cluster analysis of the PhP data revealed two distinct clusters (G1 and G2) and eight single isolates (Fig.). Except for two isolates, cluster G1 included only isolates belonging to the pandemic serotypes (O3:K6, O4:K68, O1:KUT, O1:K25). Pandemic serotypes O3:K6 and O4:K68 strains isolated from Southeast Asian travellers were not found to be different from the strains isolated in India. Cluster G2 contained only strains isolated before 1996; all but one of which were serotype O3:K6. The other non-pandemic isolates showed a high diversity and each belonged to a unique PhP type. For comparative purposes, earlier presented data on the presence of toxR, tdh and trh genes by PCR assay [29, 30] and data on group-specific PCR (GS-PCR) and PCR for open reading frame ORF8 [19, 31] are also presented in the Figure. It was shown that the non-pandemic isolates are mostly negative for GS-PCR and ORF8, compared to pandemic isolates.
Fig.
UPGMA clustering of the PhP 48 typing data for 58 isolates of various serotypes of Vibrio parahaemolyticus, together with data on isolation source, isolation year, serotype and presence of toxin genes. First column shows pandemic status of the isolates, i.e. NP indicates non-pandemic, P indicates pandemic, IP indicates international pandemic strains. The last column shows the reference number for other studies that have used the same strains. NT indicates not tested. For comparative purposes, earlier presented data on the presence of toxR, tdh and trh genes by PCR assay [29, 30] and data on group-specific PCR (GS-PCR) and PCR for open reading frame ORF8 [19, 31] are shown.
In concurrence with the phenotyping data, molecular typing with PFGE of 139 V. parahaemolyticus isolates of O3:K6 serotype showed that strains isolated after 1996 from different Asian countries were closely related (strains KX-V225, 226, 231 are included in this study) and that they were distinct from O3:K6 strains isolated before 1996 (strains AQ3732, AQ4733, AQ3794, AQ4019 are included in this study) [18]. Another study by Yeung et al. (2002) also showed similar PFGE patterns of O3:K6 isolates originating from different countries (strains KX-V225, AN5034, AN16000 are included in this study) but that the pattern was distinct from non-O3:K6 strains [16]. Similarly, it has been shown that strains of the pandemic serotypes O3:K6, O4:K68 and O1:KUT exhibit a unique profile by arbitrarily primed PCR profiling, ribotyping and PFGE analysis (O3:K6 serotype strains KX-V225, KX-V226, KX-V231; O1:KUT serotype strains VP250, AN16000; O4:K68 serotype strains AN5034, KX-V508, KX-V563 are included in this study) [21]. The biochemical fingerprinting in this study also showed that V. parahaemolyticus pandemic clonal serotypes have a similar phenotypic pattern as demonstrated by molecular typing methods. The results from our study again indicate that isolates belonging to serotypes O3:K6, O4:K68, O1:KUT and O1:K25 are related in both phenotypic and genotypic characteristics and may belong to the pandemic clonal group of V. parahaemolyticus. The high phenotypic and genotypic homogeneity may indicate that the strains have diverged from a common ancestor by the alteration of genes associated with a few O:K antigens and a few single clones may be responsible for the emergence of the pandemic serotypes.
Biochemical fingerprinting, PhP typing, is a phenotyping method that is easy to learn and does not require access to expensive equipment and reagents. It can thus be used as a tool for epidemiological surveillance in laboratories where there is a lack of genotyping resources. A main advantage is that it is simple to perform and data processing and presentation are automated. In general less than 8 h of labour is required to assay 200 isolates. Furthermore, preliminary results may be obtained after overnight incubation. Thus, the PhP typing method may be suitable as a screening method for surveillance purposes and/or to study outbreaks of V. parahaemolyticus gastroenteritis, especially for large numbers of isolates, for which it can be used to select those isolates that require further typing by molecular typing methods. In conclusion, biochemical fingerprinting using the PhP system showed good agreement with molecular characterization of pandemic and non-pandemic isolates of V. parahaemolyticus.
ACKNOWLEDGEMENTS
This work was supported by SIDA/SAREC grant no. 1999-255, for a Ph.D. fellowship for Mokhlasur Rahman, and the Karolinska Institutet Fund.
DECLARATION OF INTEREST
None.
REFERENCES
- 1.Fujinoo TOY et al. On the bacteriological experimentation of shirasu-food poisoning. Medical Journal of Osaka University. 1953;4:299–304. [Google Scholar]
- 2.Daniels NA et al. Vibrio parahaemolyticus infections in the United States, 1973–1998. Journal of Infectious Diseases. 2000;181:1661–1666. doi: 10.1086/315459. [DOI] [PubMed] [Google Scholar]
- 3.Chiou CS et al. Vibrio parahaemolyticus serovar O3:K6 as cause of unusually high incidence of food-borne disease outbreaks in Taiwan from 1996 to 1999. Journal of Clinical Microbiology. 2000;38:4621–4625. doi: 10.1128/jcm.38.12.4621-4625.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Laohaprertthisan V et al. Prevalence and serodiversity of the pandemic clone among the clinical strains of Vibrio parahaemolyticus isolated in southern Thailand. Epidemiology and Infection. 2003;130:395–406. [PMC free article] [PubMed] [Google Scholar]
- 5.Okuda J et al. Emergence of a unique O3:K6 clone of Vibrio parahaemolyticus in Calcutta, India, and isolation of strains from the same clonal group from Southeast Asian travelers arriving in Japan. Journal of Clinical Microbiology. 1997;35:3150–3155. doi: 10.1128/jcm.35.12.3150-3155.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Joseph SW, Colwell RR, Kaper JB. Vibrio parahaemolyticus and related halophilic vibrios. Critical Reviews in Microbiology. 1983;10:7–123. doi: 10.3109/10408418209113506. [DOI] [PubMed] [Google Scholar]
- 7.Centers for Disease Control and Prevention (CDC) Outbreak of Vibrio parahaemolyticus infection associated with eating raw oysters. Pacific northwest, 1997–1998. Morbidity and Mortality Weekly Report. 1998;47:457–462. [PubMed] [Google Scholar]
- 8.Centers for Disease Control and Prevention (CDC) Outbreak of Vibrio parahaemolyticus infection associated with eating raw oysters and clams harvested from Long Island Sound, Connecticut, New Jersey and New York, 1998. Morbidity and Mortality Weekly Report. 1999;48:48–51. [PubMed] [Google Scholar]
- 9.Bag PK et al. Clonal diversity among recently emerged strains of Vibrio parahaemolyticus O3:K6 associated with pandemic spread. Journal of Clinical Microbiology. 1999;37:2354–2357. doi: 10.1128/jcm.37.7.2354-2357.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vuddhakul V et al. Isolation of a pandemic O3:K6 clone of a Vibrio parahaemolyticus strain from environmental and clinical sources in Thailand. Applied and Environmental Microbiology. 2000;66:2685–2689. doi: 10.1128/aem.66.6.2685-2689.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tada J et al. Detection of thermostable direct haemolysin gene (thd) and the thermostable direct hemolysin related hemolysin gene (trh) of Vibrio parahaemolyticus by polymerase chain reaction. Molecular and Cellular Probes. 1992;6:477–487. doi: 10.1016/0890-8508(92)90044-x. [DOI] [PubMed] [Google Scholar]
- 12.Kishishita M et al. Sequence variation in the thermostable direct hemolysin-related hemolysin (trh) gene of Vibrio parahaemolyticus. Applied and Environmental Microbiology. 1992;58:2449–2457. doi: 10.1128/aem.58.8.2449-2457.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shirai H et al. Molecular epidemiologic evidence for association of thermostable direct hemolysin (TDH) and TDH-related hemolysin of Vibrio parahaemolyticus with gastroenteritis. Infection and Immunity. 1990;58:3568–3573. doi: 10.1128/iai.58.11.3568-3573.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Iguchi T, Kondo S, Hisatsune K. Vibrio parahaemolyticus O serotypes from O1 to O13 all produce R-type lipopolysaccharide:SDS-PAGE and compositional sugar analysis. FEMS Microbiology Letters. 1995;130:287–292. doi: 10.1111/j.1574-6968.1995.tb07733.x. [DOI] [PubMed] [Google Scholar]
- 15.Okuda J et al. Analysis of the thermostable direct hemolysin (tdh) gene and the tdh-related hemolysin (trh) genes in urease-positive strains of Vibrio parahaemolyticus isolated on the West Coast of the United States. Journal of Clinical Microbiology. 1997;35:1965–1971. doi: 10.1128/jcm.35.8.1965-1971.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yeung PS et al. Comparative phenotypic, molecular, and virulence characterization of Vibrio parahaemolyticus O3:K6 isolates. Applied and Environmental Microbiology. 2002;68:2901–2909. doi: 10.1128/AEM.68.6.2901-2909.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chowdhury NR et al. Molecular evidence of clonal Vibrio parahaemolyticus pandemic strains. Emerging Infectious Diseases. 2000;6:631–636. doi: 10.3201/eid0606.000612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wong HC et al. Characteristics of Vibrio parahaemolyticus O3:K6 from Asia. Applied and Environmental Microbiology. 2000;66:3981–3986. doi: 10.1128/aem.66.9.3981-3986.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bhuiyan NA et al. Prevalence of the pandemic genotype of Vibrio parahaemolyticus in Dhaka, Bangladesh, and significance of its distribution across different serotypes. Journal of Clinical Microbiology. 2002;40:284–286. doi: 10.1128/JCM.40.1.284-286.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chang B et al. A unique and common restriction fragment pattern of the nucleotide sequences homologous to the genome of vf33, a filamentous bacteriophage, in pandemic strains of Vibrio parahaemolyticus O3:K6 O4:K68, and O1:K untypeable. FEMS Microbiology Letters. 2000;192:231–236. doi: 10.1111/j.1574-6968.2000.tb09387.x. [DOI] [PubMed] [Google Scholar]
- 21.Chowdhury NR et al. Clonal dissemination of Vibrio parahaemolyticus displaying similar DNA fingerprint but belonging to two different serovars (O3:K6 and O4:K68) in Thailand and India. Epidemiology and Infection. 2000;125:17–25. doi: 10.1017/s0950268899004070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Okura M et al. Genotypic analyses of Vibrio parahaemolyticus and development of a pandemic group-specific multiplex PCR assay. Journal of Clinical Microbiology. 2003;41:4676–4682. doi: 10.1128/JCM.41.10.4676-4682.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kuhn I et al. Characterization of Aeromonas spp. isolated from humans with diarrhea, from healthy controls, and from surface water in Bangladesh. Journal of Clinical Microbiology. 1997;35:369–373. doi: 10.1128/jcm.35.2.369-373.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rahman M et al. Identification and characterization of pathogenic Aeromonas veronii biovar sobria associated with epizootic ulcerative syndrome in fish in Bangladesh. Applied and Environmental Microbiology. 2002;68:650–655. doi: 10.1128/AEM.68.2.650-655.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pedersen K et al. Clonality of Vibrio anguillarum strains isolated from fish from the Scandinavian countries, Sweden, Finland and Denmark. Journal of Applied Microbiology. 1999;86:337–347. doi: 10.1046/j.1365-2672.1999.00658.x. [DOI] [PubMed] [Google Scholar]
- 26.Ansaruzzaman M et al. Clonal groups of enteropathogenic Escherichia coli isolated in case-control studies of diarrhoea in Bangladesh. Journal of Medical Microbiology. 2000;49:177–185. doi: 10.1099/0022-1317-49-2-177. [DOI] [PubMed] [Google Scholar]
- 27.Kuhn I et al. A 4-year study of the diversity and persistence of coliforms and Aeromonas in the water of a Swedish drinking water well. Canadian Journal of Microbiology. 1997;43:9–16. doi: 10.1139/m97-002. [DOI] [PubMed] [Google Scholar]
- 28.Kuhn I, Iversen A, Mollby R. The PhenePlate system for studies of the diversity of enterococcal populations from the food chain and the environment. International Journal of Food Microbiology. 2003;88:189–196. doi: 10.1016/s0168-1605(03)00179-x. [DOI] [PubMed] [Google Scholar]
- 29.Kim YB et al. Identification of Vibrio parahaemolyticus strains at the species level by PCR targeted to the toxR gene. Journal of Clinical Microbiology. 1999;37:1173–1177. doi: 10.1128/jcm.37.4.1173-1177.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Suthienkul O et al. Restriction fragment length polymorphism of the tdh and trh genes in clinical Vibrio parahaemolyticus strains. Journal of Clinical Microbiology. 1996;34:1293–1295. doi: 10.1128/jcm.34.5.1293-1295.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Matsumoto C et al. Pandemic spread of an O3:K6 clone of Vibrio parahaemolyticus and emergence of related strains evidenced by arbitrarily primed PCR and toxRS sequence analyses. Journal of Clinical Microbiology. 2000;38:578–585. doi: 10.1128/jcm.38.2.578-585.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Möllby R, Kühn I, Katouli M. Computerized biochemical fingerprinting: a new tool for typing of bacteria. Reviews in Medical Microbiology. 1993;56:1999–2006. [Google Scholar]