SUMMARY
We determined the prevalence and risk factors of H. pylori infection among 197 healthy 3- to 5-year-old Israeli Arab children, in a population under socioeconomic and environmental transition. Data on the socioeconomic and environmental characteristics were obtained by personal interviews. The presence of H. pylori infection was identified using an ELISA kit for detection of H. pylori antigens in stool specimens. The prevalence rate of H. pylori infection was 49·7% (95% CI 42·8–56·67). It varied significantly among the different villages. In the univariate analysis stratified by village, the risk of infection increased according to household crowding, number of siblings younger than 5 years and siblings' H. pylori positivity. In the multivariate analysis the village of residence and siblings' H. pylori positivity were the only variables that remained strongly associated with H. pylori infection. In a population such as that described in this study the socioeconomic and living conditions are major risk factors of H. pylori infection and the intra-familial transmission of H. pylori in early childhood has an important role.
INTRODUCTION
H. pylori infection occurs worldwide and it is estimated that about 50% of the world's population is infected with the pathogen [1, 2]. The prevalence rates of H. pylori infection are higher in developing countries than in industrialized countries [1]. H. pylori is associated with gastric disorders, is responsible for the majority of duodenal and gastric ulcers, and is classified as grade one carcinogen in gastric cancer [1–4]. Acquiring H. pylori infection is most intensive during childhood [5], and clearly, it occurs earlier in life in developing countries than in developed countries [6, 7]. Having a low socioeconomic status, living in overcrowded conditions, and having a low maternal education are associated with an increased risk of H. pylori infection [6, 8–11].
Reports from Israel indicate seroprevalence rates of H. pylori infection of 46% among young adults, and 72% among residents of rural communities aged ⩾30 years [4, 12]. There are no published data on the epidemiology of H. pylori infection among healthy paediatric populations, either Jewish or Arab, in Israel.
We carried out the present study among the Israeli Arabs who form a unique population at an advanced stage of epidemiological transition with a significant decline in infant mortality rates and a clear shift of mortality patterns from infectious diseases to chronic diseases [13]. We assessed the prevalence and potential risk factors of H. pylori infection among healthy 3- to 5-year-old Israeli Arab children living in three villages in the Meshulash region of Israel. We tried to answer the question whether the epidemiological characteristics of H. pylori infection in populations under epidemiological and environmental transition such as the Israeli Arab population, are different from those identified in developed or developing countries.
METHODS
Study population
The Israeli Arabs live in four separate geographic areas: the Galilee in northern Israel, the Meshulash in central Israel, the Negev in southern Israel, and in East Jerusalem. The majority live in rural areas. The Israeli Arabs have much lower educational levels and are at a lower socioeconomic status compared with the Jewish population. Substantial differences between the two groups are also observed regarding the sanitation infrastructure. Nevertheless, the Israeli Arab population is in a positive transition process, with ongoing improvement of the educational level and medical system. Israeli citizens, including Arabs, have mandatory health insurance according to national health insurance law. The coverage of vaccination in this community is over 95%.
Healthy children, aged 3–5 years, living in the rural Meshulash region of Israel, who were participating in a cohort follow-up study of enteric infections were also asked to participate in a cross-sectional study of H. pylori infection. The study children attended kindergartens from the villages of Jeser El-Zarka, Faradis, and Kfar Qaraa.
The Institution Review Board of Tel Aviv University approved the study. Written informed consent was obtained from the parents of the participating children.
Collection of data
Mothers of the study children were interviewed in Arabic using a structured questionnaire. The interviews were performed at the participants' homes, by interviewers who received standard training. The questionnaire was validated by a pilot study in the target population and included information about the following sociodemographic and environmental factors: age, gender, place of residence, monthly family income, mother's education, mother's age, father's education, father's age, number of siblings, number of siblings <5 years, number of persons living in the household, number of rooms in the household, and reported contact between houseflies and foods. A crowding index was computed using the following formula: (no. of persons living in the house)/(no. of rooms in the house).
Anthropometric measurements
Anthropometric measurements were performed by specially trained registered nurses. Body weight was measured to the nearest 0·1 kg using an analogue personal scale (calibrated before use), and height (to the nearest 0·1 cm) with a mobile stadiometer. z scores: height for age (HAZ), weight for age (WAZ), and weight for height (WHZ) were computed using Epi-Info software (CDC, Atlanta, GA, USA). The calculations were based on the 2000 CDC growth reference curves, which were primarily based on the US National Health Examination (NHES) and the National Health and Nutrition Examination Surveys (NHANES).
Collection of stool specimens
Mothers of the study children were asked to collect a stool sample from their child. For a subset of study children living in Jeser El Zarka and Faradis a stool sample was also requested from their mothers as well as the sib that was nearest to the age to the study child. Fresh specimens were obtained from subjects using collection cups. After collection, the specimens were kept and transported on ice-packs in thermally isolated boxes and within 24 h of collection, aliquoted and frozen at the research laboratory at −20°C until tested. The storage and transportation of specimens were conducted by similar means and according to the same protocol in the three villages. The whole process was supervised by a field coordinator.
Detection of H. pylori infection
A commercial enzyme linked immunoassay kit (Premier HpSA, Meridian Bioscience Inc., Cincinnati, OH, USA) employing polyclonal anti-H. pylori antibody adsorbed to microwells was used to detect H. pylori antigen in stools according to the manufacturer's instructions. Optical density values of ⩾0·120, between 0·100 and 0·119, and <0·100 were considered positive, equivocal and negative respectively.
Data management and analysis
The data obtained were managed and analysed using the following software: Excel (Microsoft), Access 2000 (Microsoft), and SPSS version 12 (SPSS Inc., Chicago, IL, USA). Data entry was carried out by two trained persons and quality control was performed at the different stages of the data management.
Differences in socioeconomic variables characterizing the three villages were examined using the χ2 test. Proportions and 95% confidence intervals (CI) were computed to estimate the prevalence rates of H. pylori. The analysis of data on the potential predictive factors was performed by stratification according to the place of residence, Jeser El Zarka vs. Faradis and Kfar Qaraa using the Mantel–Haenszel test. The data on all the tested variables were homogenous as documented by the Breslow–Day and Tarone's tests of homogeneity indicating the possibility to run this pooled analysis. For each variable pooled odds ratios (OR) of infection and 95% CI were computed. Variables that were significantly associated with H. pylori infection in the Mantel–Haenszel analysis were included in multivariate analysis using logistic regression models to study the independent effect of each variable on the risk of having H. pylori infection while controlling for other variables in the model. Adjusted OR and 95% CI were computed for each variable. Two-tailed P<0·05 was considered significant.
RESULTS
Between June 2004 and October 2004, 197 children (105 males), mean age 3·7 years (s.d.=0·55), were enrolled in the study. Fifty-four lived in Kfar Qaraa, 64 in Faradis and 79 in Jeser El Zarka. The environmental and socioeconomic characteristics of the three villages were significantly different as revealed by analysis of variables such as the mother's education, monthly family income, crowding index and reported contact of flies with food (Table 1).
Table 1.
Socioeconomic and environmental characteristics of the three villages participating in the study: Jeser El Zarka, Faradis, and Kfar Qaraa, Israel, June–September 2004
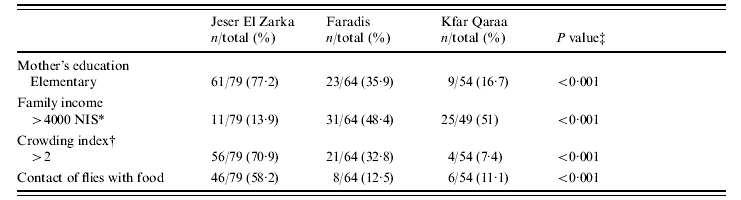
New Israeli Shekel.
Number of people living in the house divided by the number of rooms in the house.
χ2 test. Two tailed P<0·05 significant.
A total of 98 positive, three equivocal, and 96 negative stool samples for H. pylori were identified. In all further analyses, the three equivocal subjects were classified as negative.
The overall prevalence rate was 49·7% (95% CI 42·8–56·6). The prevalence rate in Jeser El Zarka was over double that of Kfar Qaraa and Faradis: 75·9% (95% CI 65·4–84) compared to 33·3% (95% CI 22·2–46·6) and 31·3% (95% CI 21·1–43·3) respectively (P<0·001).
Seventy-four mothers and 65 siblings with the mean age of 6 years (s.d.=3 years), one for each study child were also tested for H. pylori. The rate of H. pylori positivity was 82·4% among the mothers and 58·5% among the siblings. There were no significant differences between families who participated in this part of the study compared with those who did not participate according to place of residence, monthly family income, mother's education and crowding index.
In view of the substantial differences among the three villages in socioeconomic and environmental factors and the significant association between the village of residence and H. pylori infection rates, the analysis of the potential risk factors was performed with stratification by the village of residence; Jeser El Zarka vs. Faradis and Kfar Qaraa, using the Mantel–Haenszel test. This analysis revealed a significant increased risk for H. pylori infection among the study children with an increase in the crowding index, number of siblings <5 years and presence in the household of a sib with H. pylori infection (Table 2). No statistically significant differences in the prevalence rates of H. pylori infection were detected between genders and according to children's age groups, mother's education, father's education, parents' age groups, monthly family income, number of siblings, maternal H. pylori infection, and reported contact of food by houseflies (Table 2).
Table 2.
Stratified analysis of risk factors of H. pylori infection among 3- to 5-year old Israeli Arab children, from the Meshulash region, Israel, June–September 2004
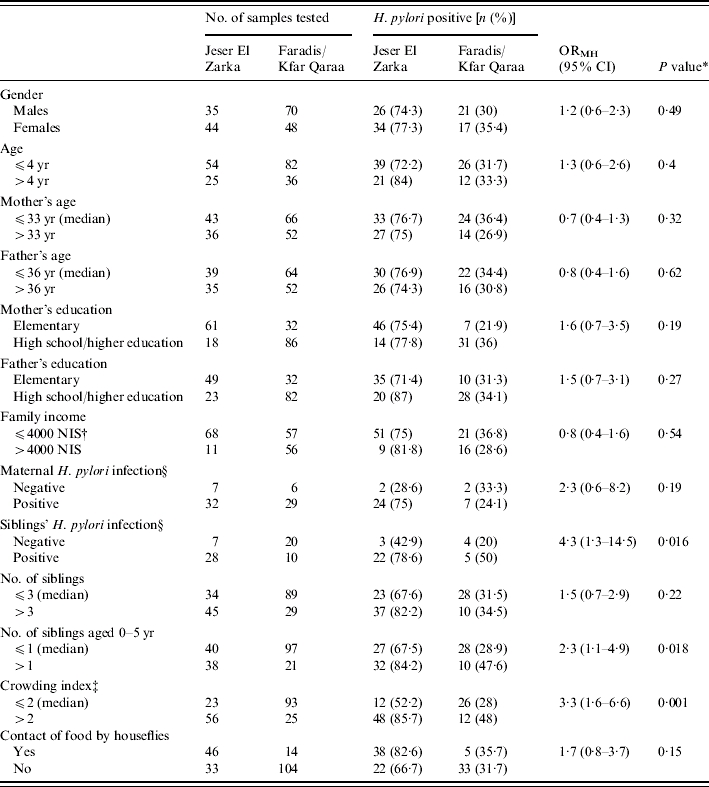
ORMH, Mantel–Haenzel odds ratio.
Mantel–Haenzel test P<0·05 significant.
New Israeli Shekel.
Number of people living in the house divided by the number of rooms in the house.
Only children from Faradis and Jeser El Zarka were included in the analysis.
An initial multivariate logistic regression analysis was carried out on data related to 196 subjects; it involved the variables that showed a statistically significant association with H. pylori positivity in the Mantel–Haenszel analysis except the variable ‘sibling's H. pylori infection’ for which information was available on 65 children.
All the variables that were included in the model retained their significant association with the risk of H. pylori infection. Children from Jeser El Zarka were at a three-fold higher risk of being infected with H. pylori than children from Faradis and Kfar Qaraa, adjusted OR 3·3 (95% CI 1·6–6·8). Children from households with a crowding index of >2 persons per room were at around a three-fold higher risk of H. pylori infection compared to children with a crowding index of <2 persons per room, adjusted OR 3·3 (95% CI 1·6, 6·7). When the number of siblings <5 years old was more than one, the risk of H. pylori infection increased to 2·4 compared with children having one or no siblings <5 years old, adjusted OR 2·4 (95% CI 1·1–5).
A second multivariate analysis was performed on the 65 subjects for whom all the variables significantly associated with H. pylori positivity in the univariate analysis were available, including the variable ‘sibling's H. pylori infection’. This model revealed that the risk of H. pylori infection was 3·3 times higher among children from Jeser El Zarka compared with children from Faradis (Table 3). Children having a sibling positive for H. pylori infection had a 4·5-fold higher risk of H. pylori infection than children with no H. pylori infection in the tested sibling (Table 3). The variables: crowding index, and number of siblings <5 years old did not retain a significant association with H. pylori infection.
Table 3.
Multivariate analysis of the association between different risk factors and H. pylori positivity among 3- to 5-year old Israeli Arab children, from the Meshulash region, Israel, June–September 2004
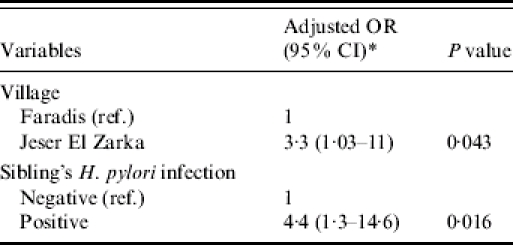
Adjusted for the variables: village, sibling's H. pylori infection, crowding index and siblings aged 0–5 years. The associations between crowding index and siblings aged 0–5 years and H. pylori infection were not significant in this model.
The association between H. pylori infection and anthropometric measurements
The anthropometric measurements were similar among H. pylori-positive and -negative subjects. The mean differences in height for age z scores (HAZ), weight for age z scores (WAZ), and weight for height z scores (WHZ) among H. pylori-positive and -negative subjects were 0·158, −0·013, and −0·166 respectively (P=0·2, 0·9, 0·2).
DISCUSSION
This community-based study was conducted among children in the Israeli Arab population, going through an ongoing process of socioeconomic and environmental transition.
To the best of our knowledge there are no published data regarding the epidemiology of H. pylori infection among healthy children, either Jewish or Arab in Israel. Using a recently developed ELISA system for direct detection of specific H. pylori antigen in stool specimens, we found high prevalence rates of H. pylori infection among healthy 3- to 5-year-old children. Data of a recent follow-up study [14] carried out among children in the United States reconfirmed the validity of the ELISA antigen detection system used in our study. Haggerty et al. [14] reported that the vast majority of the positive stool samples for H. pylori as detected by stool antigen ELISA system, were also positive by PCR. Only a few antigen-positive stools harboured Helicobacter of other species than H. pylori.
A previous article from Israel studying the seroprevalence of H. pylori antibodies reported a 46·5% H. pylori infection rate among Jewish young adults aged 18–19 years [4], similar to the overall rate found in our study in a much younger age group (preschool children). These findings may suggest an earlier acquisition of H. pylori infection among Arabs as compared to Jews in Israel.
The prevalence rate of H. pylori infection in our study is similar to rates described among children in developing countries [6, 9]. It varied significantly among the three villages, but was the highest in Jeser El Zarka. The substantial differences in the rates of H. pylori infection in these three villages can be explained by their different socioeconomic and living conditions. Variations in H. pylori infection rates in a defined geographic area were also observed in Germany and Italy but in each of these studies the participants belonged to different subpopulations, according to their nationality or regarding rural vs. urban communities [15, 16]. Our study controls for these differences and suggests that in the same ethnic group, within a small geographic area, and in a rural environment, there are groups at substantially high risk of H. pylori infection.
Living in a village of low socioeconomic status, under conditions of crowding, with a high number of young siblings and with a sibling positive for H. pylori were factors that showed a significant and independent association with an increased risk of H. pylori infection among the preschool children. Two of these predictors, namely the village of residence and carrying H. pylori within the family, emerged as being the strongest variables associated with the risk of H. pylori infection.
The importance of socioeconomic status, household crowding and intra-familial transmission regarding the risk of acquiring H. pylori infection in early childhood has been reported in a series of articles [9, 17–20]. Each of these studies controlled, however, for a limited number of potential confounders. Our study was carried out in a paediatric population attending kindergartens, homogeneous in terms of ethnicity, age, and geographic place of residence and controls for a large number of potential confounders.
The relationship between sibling's H. pylori positivity and the increased risk of H. pylori infection among the study children probably supports the possibility of intra-familial transmission rather than exposure to a common source of infection since the analysis included children from different villages.
We assume that close personal contact in families where carriers of H. pylori are already present among siblings or parents can markedly increase the risk of oral–oral, gastro–oral or faecal–oral transmission through inadvertently spreading infected saliva, vomitus or having poor personal hygiene. Different modes or mechanisms of intra-familial transmission of H. pylori have been proposed, for example, sharing a cup [20] and sharing a bed or a bedroom with a H. pylori-infected sibling in early childhood [21].
The association between the child's H. pylori infection and maternal H. pylori infection was not significant and could be explained by the small sample size and high positivity rate among mothers.
The issue of a possible association between nutritional status and H. pylori infection is controversial. This can be the result of differences in the methodology applied in the various studies including different sample sizes and the variety of the study populations among which stronger correlates of nutritional status could mask a potential specific association. In our study, anthropometric measurements were not associated with H. pylori infection. Other cross-sectional studies from Egypt and Bangladesh [9, 10, 22] reported similar findings. However, data which was obtained through a nested case control among Peruvian children, aged ⩾2 years, showed less weight gain 2 months after H. pylori antibody seroconversion [23].
In summary, in the Israeli Arab population going through an ongoing process of socioeconomic and environmental transition, the prevalence and risk factors of H. pylori infection among healthy children at the age of 3–5 years are similar to those previously reported in developing countries.
ACKNOWLEDGEMENTS
The study was supported by grant no. 01-18-00437 from the Israel Ministry of Science. The authors thank the fieldwork team: Hiba Abu-Abed, Shiraz Muhsen, Ola Abu-Shehab, Manal Jurban, Roza Marai, for their contribution in the process of data collection, and Sophie Goren for her help in the data management.
DECLARATION OF INTEREST
None.
REFERENCES
- 1.Suerbaum S, Michetti P. Helicobacter pylori Infection. New England Journal of Medicine. 2002;347:1175–1187. doi: 10.1056/NEJMra020542. [DOI] [PubMed] [Google Scholar]
- 2.Torres J et al. Comprehensive review of the natural history of Helicobacter pylori infection in children. Archives of Medical Research. 2000;31:431–469. doi: 10.1016/s0188-4409(00)00099-0. [DOI] [PubMed] [Google Scholar]
- 3.Goodwin CS, Mendall MM, Nortfield TC. Helicobacter pylori infection. Lancet. 1997;349:265–269. doi: 10.1016/S0140-6736(96)07023-7. [DOI] [PubMed] [Google Scholar]
- 4.Gdalevich M et al. Helicobacter pylori infection and subsequent peptic duodenal disease among young adults. International Journal of Epidemiology. 2000;29:592–595. [PubMed] [Google Scholar]
- 5.Mitchell A et al. Age-specific Helicobacter pylori seropositivity rates of children in an impoverished urban areas of northeast Brazil. Journal of Clinical Microbiology. 2003;41:1326–1328. doi: 10.1128/JCM.41.3.1326-1328.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Frenck RW, Clemens J. Helicobacter in the developing world. Microbes and Infection. 2003;5:705–713. doi: 10.1016/s1286-4579(03)00112-6. [DOI] [PubMed] [Google Scholar]
- 7.Malaty HM et al. Age at acquisition of Helicobacter pylori infection: a follow up study from infancy to adulthood. Lancet. 2002;16:931–935. doi: 10.1016/S0140-6736(02)08025-X. [DOI] [PubMed] [Google Scholar]
- 8.Wizla-Derambure N et al. Familial and community environmental risk factors for Helicobacter pylori infection in children and adolescents. Journal of Pediatrics and Gastroenterological Nutrition. 2001;33:58–63. doi: 10.1097/00005176-200107000-00010. [DOI] [PubMed] [Google Scholar]
- 9.Clemens J et al. Sociodemographic, hygienic and nutritional correlates of Helicobacter pylori infection of young Bangladeshi children. Pediatrics Infectious Disease Journal. 1996;15:1113–1118. doi: 10.1097/00006454-199612000-00012. [DOI] [PubMed] [Google Scholar]
- 10.Mahalanabis D et al. Helicobacter pylori infection in the young in Bangladesh: prevalence, socioeconomic and nutritional aspects. International Journal of Epidemiology. 1996;25:894–898. doi: 10.1093/ije/25.4.894. [DOI] [PubMed] [Google Scholar]
- 11.Dominici P et al. Familial clustering of Helicobacter pylori infection: population based study. British Medical Journal. 1999;319:537–540. doi: 10.1136/bmj.319.7209.537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gilboa S et al. Helicobacter pylori infection in rural settlements (Kibbutzim) in Israel. International Journal of Epidemiology. 1995;24:232–237. doi: 10.1093/ije/24.1.232. [DOI] [PubMed] [Google Scholar]
- 13.Israeli Center for Disease Control 1999. p. 45. . Ministry of Health, Health status in Israel 1999. Pitah Tikva: Ofset color,
- 14.Haggerty TD et al. Significance of transiently positive enzyme-linked immunosorbent assay results in detection of Helicobacter pylori in stool samples from children. Journal of Clinical Microbiology. 2005;43:2220–2223. doi: 10.1128/JCM.43.5.2220-2223.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rothenbacher D et al. Prevalence and determinants of Helicobacter pylori infection in preschool children: a population based study from Germany. International Journal of Epidemiology. 1998;27:135–141. doi: 10.1093/ije/27.1.135. [DOI] [PubMed] [Google Scholar]
- 16.Dore MP et al. Risk factors associated with Helicobacter pylori infection among children in a defined geographic area. Clinical Infectious Diseases. 2002;35:240–245. doi: 10.1086/341415. [DOI] [PubMed] [Google Scholar]
- 17.Goodman K, Correa P. Transmission of Helicobacter pylori among siblings. Lancet. 2000;355:358–362. doi: 10.1016/S0140-6736(99)05273-3. [DOI] [PubMed] [Google Scholar]
- 18.Rothenbacher D et al. Role of infected parents in transmission of Helicobacter pylori to their children. Pediatric Infectious Disease Journal. 2002;21:674–679. doi: 10.1097/00006454-200207000-00014. [DOI] [PubMed] [Google Scholar]
- 19.Rocha GA et al. Transmission of Helicobacter pylori infection in families of preschooled aged children from Minas Gerais, Brazil. Tropical Medicine and International Health. 2003;8:987–991. doi: 10.1046/j.1360-2276.2003.01121.x. [DOI] [PubMed] [Google Scholar]
- 20.Rodrigues MN et al. Prevalence of Helicobacter pylori infection in children from urban community in northeast Brazil and risk factors of the infection. European Journal of Gastroenterology and Hepatology. 2004;16:201–205. doi: 10.1097/00042737-200402000-00013. [DOI] [PubMed] [Google Scholar]
- 21.Farrell S et al. Risk factors of Helicobacter pylori infection in children; an examination of the role played by intrafamilial bed sharing. Pediatric Infectious Disease Journal. 2005;24:149–152. doi: 10.1097/01.inf.0000151104.14058.70. [DOI] [PubMed] [Google Scholar]
- 22.Naficy AB et al. Seroepidemiology of Helicobacter pylori infection in a population of Egyptian children. International Journal of Epidemiology. 2000;29:928–932. doi: 10.1093/ije/29.5.928. [DOI] [PubMed] [Google Scholar]
- 23.Passaro DJ et al. Growth slowing after acute Helicobacter pylori infection is age dependent. Journal of Pediatrics and Gastroenterological Nutrition. 2002;35:522–526. doi: 10.1097/00005176-200210000-00012. [DOI] [PubMed] [Google Scholar]


