SUMMARY
We analysed the characteristics of the pregnancies with a previously undetected HIV infection in a national observational study of pregnant women with HIV in Italy. In a total of 443 pregnancies with available date of HIV diagnosis, 118 were characterized by a previously undetected HIV infection (26·6%, 95% CI 22·5–30·8). The following factors were independently associated with this occurrence in a multivariate analysis (adjusted odds ratios; 95% CIs): foreign nationality (5·1, 2·8–9·3); no pre-conception counselling (35·9, 4·8–266·1); first pregnancy (2·1, 1·2–4·0); asymptomatic status (6·8, 1·5–30·6). Women with previously undetected infection started antiretroviral treatment significantly later during pregnancy (P<0·001). Missed diagnosis was responsible for one case of transmission. A high rate of previously undetected HIV infection was observed. This suggests a good HIV detection during pregnancy, but also the need to reinforce HIV testing strategies among women of childbearing age. We identified some determinants which may be considered for intervention measures.
INTRODUCTION
In developed countries the rate of vertical transmission of HIV has dramatically declined after the introduction of a combination of measures represented by antiretroviral treatment before and at delivery, elective caesarean section and elimination of breastfeeding. Such measures are strongly effective, and in many case series and clinical trials HIV transmission rates well below 5%, and sometimes close to 0%, have been reported [1–5]. However, these measures can only be applied and be effective if the woman with HIV infection is aware of being infected. Although HIV testing is always recommended during pre-conception counselling, many pregnancies, even if planned, are not preceded by a counselling visit, and a substantial proportion of pregnancies are not planned at all. For all the above reasons, it is not uncommon to have a first assessment for HIV and other infections once pregnancy is already established. A timely HIV assessment, performed in the early stages of pregnancy, is of crucial importance for a complete preventive intervention against HIV transmission to the newborn. Late presentation during pregnancy may lead to a late diagnosis, or (if the woman with HIV is not tested even at delivery) to a missed diagnosis of HIV infection. In this condition (absence of any intervention), the risk of transmission is assumed to be about 20%, even in developed settings [6, 7].
Identifying the clinical and demographic characteristics associated with a diagnosis of HIV during pregnancy may be relevant for different reasons. First, the definition of the rate and of the clinical or demographic characteristics associated with a previously undetected HIV infection may be useful for surveillance purposes, contributing to a better understanding of the dynamics of the HIV epidemics in the population. Second, the definition of predictors for undetected HIV infection may allow the identification of targets for selective HIV testing, which might be used to enhance the cost-effectiveness of testing strategies. Selective screening strategies targeting persons who reported risk factors plus persons in high-prevalence demographic groups are expected to have superior efficacy compared to screening strategies targeting only persons with reported risk factors [8]. Discovering HIV infection may produce important individual benefits for women with undetected HIV infection and their offspring, which include the possibility of preventing important immunological decline and HIV-related opportunistic infections, a timely initiation of antiretroviral therapy, potential reductions in at-risk sexual behaviour and in horizontal transmission to non-infected partners, a more conscious planning of pregnancy, and a more timely implementation of preventive measures against vertical transmission in pregnancy. This is particularly relevant for antenatal antiretroviral treatment, which is considered to reduce the risk of transmission through the suppression of maternal viral load. Although the optimal time for starting antenatal treatment it is not known, a short period of antiretroviral treatment in pregnancy was observed for children who became infected despite maternal HAART in the European Collaborative Study [9]. These findings support the need for a timely diagnosis of HIV and for pre-conception/early screening in pregnancy.
In order to obtain additional information on this subject we used data from an ongoing national observational study on the use of antiretroviral treatment in pregnancy. The objectives of the present study were to assess the rate of previously undetected HIV infection among pregnant women with HIV, to define the clinical and demographic characteristics associated with this condition, and to explore the possible impact of a diagnosis of HIV during pregnancy on the time of start of antiretroviral therapy in pregnant women.
METHODS
The Italian Group on Surveillance on Antiretroviral Treatment in Pregnancy was established in 2001 in order to collect information on the safety and efficacy of antiretroviral treatment in pregnancy. Obstetricians, infectious diseases specialists and paediatricians participate in the programme. All pregnancies with an outcome occurring after 1 December 2001 are eligible for inclusion. Only HIV-positive pregnant women are included, and no indication is given for treatment, which is at the discretion of the treating physician. Informed consent is required for all women, using a patient information sheet approved by the competent Ethics Committee. The study is coordinated by the Istituto Superiore di Sanità (ISS), the Italian National Institute of Health. Data are collected through three different networks: (1) SIGO National Study Group on HIV Infection; (2) Gruppo Laziale Donne Gravidanza e HIV; (3), centres directly coordinated by the ISS. Data are collected at clinical centres using specifically designed case report forms and are then entered at the Coordinating Centre at ISS, where queries are also generated and analyses performed. An advisory board and all participants are regularly updated with the results of the surveillance.
All the results reported here are based on the data extracted on 25 August 2004 from the general database. For the present analysis, all the pregnancies with a known date of diagnosis of HIV were used, and for each woman only the first pregnancy reported was considered. The occurrence of a diagnosis of HIV infection during pregnancy was defined by a date of first HIV positive test after date of last menstrual period. The women with a first positive HIV test after last menstrual period were considered new diagnoses and those with a date of first positive HIV test before date of last menstrual period were considered pre-existing diagnoses. These two groups were compared for clinical and demographic characteristics, using the χ2 test for categorical variables and the t test or the Mann–Whitney U test, according to the characteristics of the distribution (symmetrical or skewed, respectively), for quantitative variables. Assumptions of normality were tested by review of plots and by the Kolmogorov–Smirnov test. Unadjusted odds ratios were calculated based on contingency tables and on univariate logistic regression models. All those factors which resulted in univariate analyses below the significance level of 0·10 were included in a multivariate logistic regression model which used the presence of a new diagnosis as the dependent variable and in which the covariates not significantly contributing to the model were progressively removed in a backward stepwise procedure. Multivariate analysis also included age as a covariate. The role of age in the model was assessed performing two separate analyses, considering age either as a continuous variable or as a categorical variable (with groups defined according to quartiles). Ninety-five percent confidence intervals for proportions and for both unadjusted and adjusted odds ratios were calculated. Time of start of treatment during pregnancy was assessed comparing week of start of treatment between groups defined by time of diagnosis (new or pre-existing). For women who were already on treatment at conception, treatment was considered to start at first week of pregnancy. The proportions of HIV-infected infants born from the groups of women with new and pre-existing diagnoses were compared through the χ2 test. All the analyses were performed using SPSS software, version 11.0.1 (SPSS Inc., Chicago, IL, USA).
RESULTS
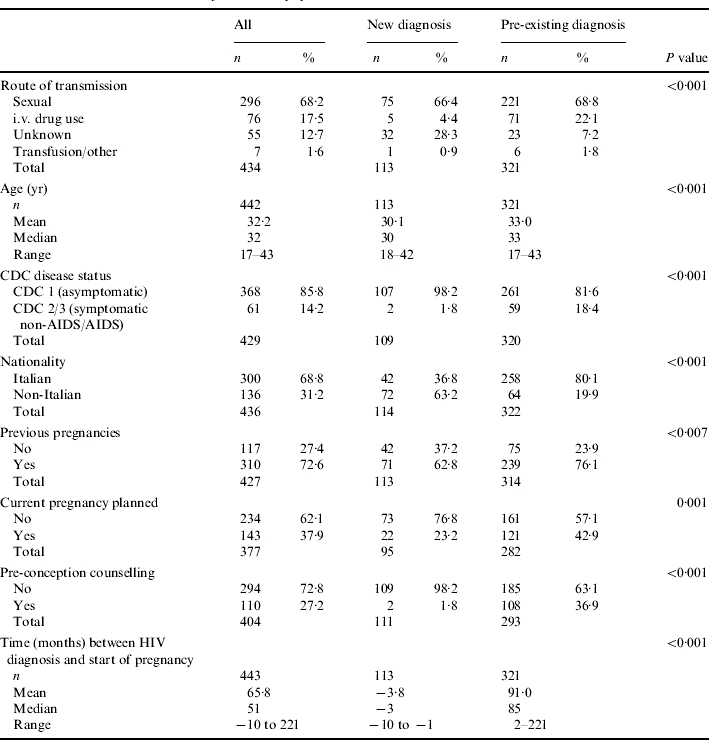
From 25 August 2004, data on time of HIV diagnosis was available for 453 pregnancies regarding 443 women. The main characteristics of these women are reported in Table 1. Overall, median age at conception was 32 years; current pregnancy was planned in only 38% of cases, and pre-conception counselling was also uncommon (27%). Most of the women (68%) were infected through the sexual route (route unknown in 13%), and at first evaluation during pregnancy 86% were still asymptomatic with respect to HIV infection. The average time from HIV diagnosis to pregnancy was 66 months (median 51 months). Among the 443 pregnancies considered, 118 were characterized by a diagnosis of HIV infection after date of last menstrual period (26·6%, 95% CI 22·5–30·8). When compared to women with a previously established diagnosis, the two groups showed important and statistically significant differences in the following characteristics (Table 1): pre-conception counselling (new diagnosis: 1·8%; pre-existing diagnosis: 36·9%, P<0·001); nationality (non-Italian: new diagnosis 63·2%; pre-existing diagnosis 19·9%, P<0·001); Centers for Disease Control (CDC) group (asymptomatic: new diagnosis 98·2%; pre-existing diagnosis 81·6%, P<0·001); current pregnancy planned (new diagnosis: 23·2; pre-existing diagnosis: 42·9%, P=0·001); previous pregnancies (new diagnosis: 62·8%; pre-existing diagnosis: 76·1%, P=0·007). The two groups were also significantly different in terms of age, with a lower age in the group of women with a new diagnosis (mean difference: 2·9 years, 95% CI of the difference: 1·9–3·9 years; P<0·001) and in the distribution of route of transmission of HIV infection (P<0·001). Women who reported a previous intravenous (i.v.) drug use as the likely route of transmission had a significantly higher probability to have HIV infection diagnosed prior to pregnancy (odds ratio with reference to women with a sexual route: 4·82, 95% CI 1·8–12·4, P=0·001); conversely, women with a reported unknown route of transmission had a lower probability to be already aware of being infected (OR 0·24, 95% CI 0·13–0·44, P=0·001).
Table 1.
General characteristics of the entire population
When all the factors achieving statistical significance were included in the multivariate analysis, the model progressively removed route of transmission, pregnancy planned and age, as non-significant covariates. The effect of age was not significant in two separate multivariate analyses based on age both as a continuous and as a categorical variable. The covariates significantly related to a diagnosis of HIV in pregnancy were absence of pre-conception counselling, asymptomatic status (CDC group 1), foreign nationality and first pregnancy. Adjusted odds ratio with 95% confidence intervals for these variables are reported in Table 2.
Table 2.
Factors significantly related to a diagnosis of HIV infection during pregnancy
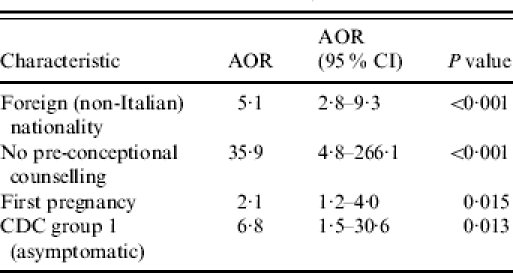
AOR, Adjusted odds ratio; CI, confidence interval.
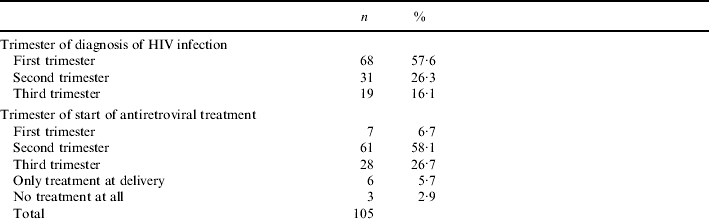
Among women with previously undetected infection, the new diagnosis occurred in the majority of cases in the first trimester, although 16% of diagnoses occurred at third trimester (Table 3). Time of diagnosis had an important effect on time of start of antiretroviral treatment during pregnancy: most of the women with a pre-existing diagnosis were already on treatment at the start of pregnancy (week 1: 182/283, 64%). In the other group, the proportion of women on antiretroviral therapy increased progressively during pregnancy, reflecting the time of diagnosis (Table 3). Median week of start of treatment was week 1 in the group with a pre-existing diagnosis (interquartile range 1–14) and week 22 (interquartile range 16–28) in the women with a new diagnosis. This difference was highly significant (P<0·001, Mann–Whitney U test), indicating a substantial delay in start of treatment among women with a new diagnosis. The proportion of women starting treatment at each trimester, together with other demographic characteristics of the women with a new diagnosis, is shown in Table 3.
Table 3.
Other characteristics of women with a diagnosis of HIV in pregnancy
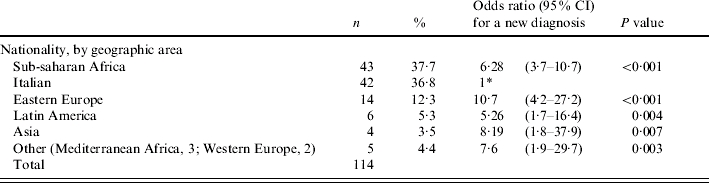
Reference group.
Follow-up data on HIV transmission was available for 112/443 pregnancies (25%), 77 from the pre-existing diagnosis group (23·7%) and 35 from the new diagnosis group (29%). Three cases of transmission were observed (2·7%), two in the group of the women with a new diagnosis (5·7%) and one in the group with a pre-existing diagnosis (1·3%). The difference between the two groups was not significant (P=0·23). In the group of women with a new diagnosis who transmitted infection, one received no antiretroviral treatment at all during pregnancy due to diagnosis occurring after delivery, and the other started treatment at 34 weeks of pregnancy. In the group of women with a pre-existing diagnosis, the woman who transmitted HIV to the newborn had received HAART throughout pregnancy (with an interruption from week 10 to week 16), but harboured a multidrug-resistant strain of HIV.
DISCUSSION
Recent reports indicate that vertical transmission of HIV still occurs in industrialised countries as a consequence of inadequate prenatal care. Factors related to HIV transmission in such settings include absence of prenatal care, unawareness of being HIV-infected, low adherence with treatment prescriptions and no treatment or no HIV testing until labour [10]. A late diagnosis of HIV during pregnancy may compromise optimal therapeutic management and affect the risk of transmission. It is, therefore, particularly important to define the frequency and characteristics of this occurrence, because such information may be used to identify target groups and to develop or expand preventive measures and intervention strategies in prenatal care.
In our observational study, which represents a large national survey of pregnant women with HIV infection, more than a hundred new diagnoses in pregnant women were reported in around 2 years, corresponding to a proportion of 26% among the pregnant women with HIV in our cohort.
From these data we cannot draw conclusions on the general prevalence of HIV infection among women of childbearing age, because our cohort evaluates only HIV-positive women. Nonetheless, these figures are alarming, and they clearly point to the need to further explore this issue with prevalence studies among unselected pregnant women to better define the current epidemiological trends in HIV infection. It is important to note that most of the women enrolled had had previous pregnancies and that some of the women here classified as having pre-existing diagnoses may also have had HIV diagnosed during a past pregnancy; the occurrence of an HIV diagnosis during pregnancy may, therefore, be even more common.
Our data indirectly suggest that voluntary HIV testing among sexually active women is often delayed until pregnancy occurs. A high rate of detection of HIV during pregnancy, although alarming, may indicate that even if few women may undergo an HIV test before pregnancy, antenatal screening is effective in identifying a substantial number of infections. Antenatal testing for HIV is recommended for all pregnant women and is free of charge in Italy in the public setting. Policy for testing is based on an opt-in approach, and the uptake of the test is likely to be incomplete. Limited information is available on that subject. Our study, being based on HIV-positive women only, does not allow us to establish the uptake of testing during pregnancy and the proportion of missed diagnoses during pregnancy. However, we quite commonly observed a history of previous pregnancies among women with a new diagnosis (63%), a condition that may indicate either new acquisition of HIV in women tested and found HIV-negative in previous pregnancies or a missed diagnosis in past pregnancies, which clearly represents a source of concern.
In our cohort, four factors were found to be independently related to the occurrence of a first HIV-positive test during pregnancy: foreign nationality, no pre-conception counselling, first pregnancy and asymptomatic status.
Asymptomatic status and absence of previous pregnancies are likely to represent two markers of overall good conditions and possibly of few previous contacts with health services. Similarly, the relation between a new diagnosis of HIV in pregnancy and foreign nationality might reflect limited access prior to pregnancy to prevention services and to some aspects of medical care, including HIV testing, for these women, or the inability of existing informative campaigns about HIV testing to reach women of a foreign nationality.
Particular attention should be paid to women with such characteristics and specific strategies should be developed to increase the overall number of women who have a pre-conception counselling visit, because its absence proved to be the most significantly predictive factor of a new diagnosis of HIV during pregnancy.
Pre-conception counselling (here defined broadly as advice on multiple health issues regarding pregnancy, including HIV) has important potential benefits with respect to HIV infection: it is expected to increase the rate of testing among women never tested before, allowing use of effective preventive measures in newly diagnosed women (antiretroviral treatment, mode of delivery, and avoidance of breastfeeding) and to lead to proper prenatal care among women already aware of their HIV infection, with positive and important effects on both maternal and child health. These benefits include better control of maternal HIV disease during pregnancy, review of antiretroviral therapy with selection of safer regimens, and multidisciplinary management of infected pregnant women [11–13].
A timely diagnosis of HIV is particularly relevant, because a missed or a late diagnosis may lead to HIV transmission [10]. Even women arriving at the end of pregnancy with an unknown HIV status may be tested a few days before delivery or during labour, allowing use of intrapartum therapy in the mother and neonatal prophylaxis of HIV-exposed newborns with zidovudine or other antiretroviral drugs [10].
In our study, women with a new diagnosis started antiretroviral treatment significantly later compared to women with a pre-existing diagnosis. Most of the women with a new diagnosis started antiretroviral treatment at second trimester (at a median gestational time of 22 weeks), a time that can be compatible with an effective protection against intrapartum HIV transmission. However, in about one third of the new diagnoses the time of start of treatment suggests a lower degree of protection against transmission: 27% of the women started treatment at third trimester, 6% had antiretroviral treatment only at delivery, and 3% (three cases) had no treatment at all. The reasons for not administering any antiretroviral treatment in these three women were: missed diagnosis during pregnancy, premature delivery at 25 weeks, and late presentation with HIV testing during an emergency delivery.
In our cohort study, two women with a late (34 weeks) or missed diagnosis transmitted infection to the newborn. We are currently unable to define precisely the impact of a late or missed diagnosis on transmission because our follow-up data on HIV transmission is still being collected and the overall rate of transmission is low. Nonetheless, we believe that all efforts should be taken to avoid a late or a missed diagnosis of HIV infection in pregnancy. This may be obtained by strengthening HIV testing strategies directed to non-pregnant women of childbearing age and by strongly recommending an early HIV assessment for all pregnant women.
Several studies have addressed HIV testing and found it cost-effective in different settings. Recent studies support the cost-effectiveness of HIV screening in the general population and in ‘high risk’ populations [14, 15]. Decision models have shown that an enhanced prenatal screening programme would avert a significant number of newborn infections and provide the woman with the health benefits of antiretroviral treatment at a low cost-effectiveness ratio [16]. Although a universal and early antenatal testing for HIV might be adequate against HIV transmission, a concomitant increase in the proportion of non-pregnant women tested could provide wider benefits, as discussed above. In this scenario, the factors that we identified could be considered in the design of targeted intervention measures directed to increase voluntary testing among women of childbearing age, with potential benefits for both maternal and child health.
ACKNOWLEDGEMENTS
We thank Cosimo Polizzi for providing excellent technical support for the study and Tonino Sofia for providing comments and help in the revision of the final manuscript. Funding was supplied by the Italian National Programme on Research on AIDS (grant nos. 39C/A, 31D55, 31D56).
APPENDIX. The Italian Group on Surveillance on Antiretroviral Treatment in Pregnancy
Project coordinators: M. Floridia, M. Ravizza, E. Tamburrini. Participants: E. Tamburrini, M. Ravizza, P. Ortolani, E. R. dalle Nogare, G. Sterrantino, M. Meli, M. Mazzetti, B. Borchi, E. Pinter, E. Anzalone, L. Roberti, A. Carocci, E. Grilli, A. Maccabruni, A. Moretti, G. Natalini, G. Guaraldi, C. Vanzini, F. Sabbatini, A. Zoncada, A. Degli Antoni, A. Molinari, P. Rogasi, M. P. Crisalli, F. Mori, E. Chiesa, G. Placido, M. Dalessandro, S. Alberico, M. Bernardon, A. Meloni, A. Citernesi, M. F. Ravagni Probizer, A. Vimercati, B. Guerra, M. Sansone, C. Tibaldi, A. Calcagno, S. Marini, G. Masuelli, L. Di Lenardo, E. Ferrazzi, V. Conserva, T. Brambilla, E. Rubino, A. Bucceri, R. Matrone, G. Scaravelli, G. Anzidei, R. Cavallini, C. Fundarò, O. Genovese, C. Cafforio, C. Pinnetti, G. Liuzzi, V. Tozzi, P. Massetti, M. Anceschi, A. M. Casadei, F. Montella, A. F. Cavaliere, M. Finelli, C. Riva, M. Cellini, V. Venturi, M. Lanari, S. Garetto, G. Castelli Gattinara, L. Mangiarotti, M. Ierardi, S. Foina, B. Salerio, S. Dalzero, A. Mattei, C. Polizzi, E. Germinario, M. F. Pirillo, R. Amici, C. M. Galluzzo, M. Floridia. Advisory Board: A. Cerioli, M. De Martino, P. Mastroiacovo, M. Moroni, F. Parazzini, G. Pardi, E. Tamburrini, S. Vella. SIGO-HIV Group national coordinators: E. Ferrazzi, P. Martinelli.
DECLARATION OF INTEREST
None.
REFERENCES
- 1.Cooper ER et al. Combination antiretroviral strategies for the treatment of pregnant HIV-1-infected women and prevention of perinatal HIV-1 transmission. Journal of Acquired Immune Deficiency Syndrome. 2002;29:484–494. doi: 10.1097/00126334-200204150-00009. [DOI] [PubMed] [Google Scholar]
- 2.Dorenbaum A et al. Two-dose intrapartum/newborn nevirapine and standard antiretroviral therapy to reduce perinatal HIV transmission: a randomized trial. Journal of the American Medical Association. 2002;288:189–198. doi: 10.1001/jama.288.2.189. [DOI] [PubMed] [Google Scholar]
- 3.Bucceri AM et al. Combination antiretroviral therapy in 100 HIV-1-infected pregnant women. Human Reproduction. 2002;17:436–441. doi: 10.1093/humrep/17.2.436. [DOI] [PubMed] [Google Scholar]
- 4.The International Perinatal HIV Group. The mode of delivery and the risk of vertical transmission of human immunodeficiency virus type 1 – a meta-analysis of 15 prospective cohort studies. New England Journal of Medicine. 1999;340:977–987. doi: 10.1056/NEJM199904013401301. [DOI] [PubMed] [Google Scholar]
- 5.Shapiro Det al. Mother-to-child transmission rates according to antiretroviral therapy, mode of delivery, and viral load (PACTG 367) [Abstract 114]. 9th Conference on Retroviruses and Opportunistic Infections24–28. February 2002Seattle, WA [Google Scholar]
- 6.Newell ML et al. Vertical transmission of HIV-1: maternal immune status and obstetric factors. The European Collaborative Study. AIDS. 1996;10:1675–1681. [PubMed] [Google Scholar]
- 7.Connor EM et al. Reduction of maternal-infant transmission of human immunodeficiency virus type 1 with zidovudine treatment. Pediatric AIDS Clinical Trials Group Protocol 076 Study Group. New England Journal of Medicine. 1994;331:1173–1180. doi: 10.1056/NEJM199411033311801. [DOI] [PubMed] [Google Scholar]
- 8.Chou R et al. US Preventive Services Task Force. Screening for HIV: a review of the evidence for the U.S. Preventive Services Task Force. Annals of Internal Medicine. 2005;143:55–73. doi: 10.7326/0003-4819-143-1-200507050-00010. [DOI] [PubMed] [Google Scholar]
- 9.European Collaborative Study. Mother-to-child transmission of HIV infection in the era of highly active antiretroviral therapy. Clinical Infectious Diseases. 2005;40:458–465. doi: 10.1086/427287. [DOI] [PubMed] [Google Scholar]
- 10.CDC. Prenatal HIV testing and antiretroviral prophylaxis at an urban hospital – Atlanta, Georgia, 1997–2000. Morbidity and Mortality Weekly Report. 2004;52:1245–1248. [PubMed] [Google Scholar]
- 11.Coll O et al. Pregnancy and HIV infection: a European consensus on management. AIDS. 2002;16:S1–S18. (Suppl. 2): [PubMed] [Google Scholar]
- 12.Public Health Service Task Force http://aidsinfo.nih.gov/ContentFiles/PerinatalGL.pdf. http://aidsinfo.nih.gov/ContentFiles/PerinatalGL.pdf . Recommendations for use of antiretroviral drugs in pregnant HIV-1 infected women for maternal health and interventions to reduce perinatal HIV-1 transmission in the United States [online]. ( ). Accessed 19 December 2005. [PubMed]
- 13.European Collaborative Study. Pregnancy-related changes in the longer-term management of HIV-infected women in Europe. European Journal of Obstetrics and Gynecology and Reproductive Biology. 2003;111:3–8. doi: 10.1016/s0301-2115(03)00153-2. [DOI] [PubMed] [Google Scholar]
- 14.Sanders GD et al. Cost-effectiveness of screening for HIV in the era of highly active antiretroviral therapy. New England Journal of Medicine. 2005;352:570–585. doi: 10.1056/NEJMsa042657. [DOI] [PubMed] [Google Scholar]
- 15.Paltiel AD et al. Expanded screening for HIV in the United States – an analysis of cost-effectiveness. New England Journal of Medicine. 2005;352:586–595. doi: 10.1056/NEJMsa042088. [DOI] [PubMed] [Google Scholar]
- 16.Zaric GS et al. The cost effectiveness of voluntary prenatal and routine newborn HIV screening in the United States. Journal of Acquired Immune Deficiency Syndrome. 2000;25:403–416. doi: 10.1097/00042560-200012150-00004. [DOI] [PubMed] [Google Scholar]


