SUMMARY
Eighty-one adult patients with Salmonella enterica serotype Choleraesuis (S. Choleraesuis) bacteraemia treated at a university hospital from 1996 to 2004 were evaluated. Multivariate analysis with a logistic regression model was used to characterize risk factors for primary bacteraemia and mycotic aneurysm and to determine the association of clinical characteristics of patients based on ciprofloxacin susceptibility of the causative organism. The incidence per 100 000 discharges was 0·76 in 1996 and 3·9 in 2004. The overall rate of ciprofloxacin resistance among these isolates was 59% (87 isolates) and the annual rate increased with time from 0% prior to 2000 to 80% in 2004. Among these patients, 48 (59%) had primary bacteraemia and 13 (16%) had secondary bacteraemia with mycotic aneurysm. Seventy (86%) patients had fever at presentation, 22 (27%) developed shock during hospitalization, and eight (10%) died of S. Choleraesuis bacteraemia. Patients with immunocompromised conditions had a higher risk of developing primary bacteraemia (OR 18·442, P<0·001). Hypertension (OR 15·434, P=0·002) and male gender (OR 7·422, P=0·039) were associated with mycotic aneurysm. Patients with mycotic aneurysm were more frequently infected with ciprofloxacin-susceptible isolates (P=0·028) and ciprofloxacin-susceptible isolates were also more frequently associated with recurrent infection than ciprofloxacin-resistant isolates (P=0·038). The incidence of S. Choleraesuis bacteraemia has increased in the past 8 years, and this increase is associated with the upsurge of ciprofloxacin-resistant isolates.
INTRODUCTION
Over the past decade, Salmonella enterica serotype Choleraesuis (S. Choleraesuis) infection had become a serious threat to public health in Taiwan [1, 2]. S. Choleraesuis infection is highly invasive and usually associated with bacteraemia and metastatic infection in humans [1–3]. Of particular concern is the emergence of antimicrobial resistance among S. Choleraesuis at the same time the increasing incidence of S. Choleraesuis infection is reported [3–6]. As its clinical importance has increased, there have been many investigations towards mechanisms of virulence, drug resistance and risk factors for S. Choleraesuis infection. The Surveillance of Multicenter Antimicrobial Resistance in Taiwan (SMART) programme conducted in 2001 reported that ciprofloxacin resistance was found in 7·5% of S. Choleraesuis throughout Taiwan [3]. This programme also documented genetic similarity of ciprofloxacin-resistant S. Choleraesuis strains from pigs and humans [3]. Despite advances in diagnostic modalities and treatment, S. Choleraesuis infection continues to cause significant morbidity and mortality.
This retrospective analysis aimed to define and access the clinical risk factors associated with S. Choleraesuis infection in adult patients during 1996 and 2004 when drug resistance in this organism rapidly emerged.
MATERIAL AND METHODS
Patients
A total of 81 adult patients (age ⩾18 years) with S. Choleraesuis bacteraemia were identified from 1996 to 2004. These patients were treated at National Taiwan University Hospital, a university-affiliated hospital with a 2000-bed capacity located in northern Taiwan. Data on demographic characteristics, underlying disease, recent hospitalization in the 3 months prior to presentation, clinical manifestations of the patients, and antibiotics susceptibility of S. Choleraesuis isolates from these patients were retrospectively analysed.
Definitions
Patients were considered to have a localized infection when clinical, radiological, or pathological evidence was attained. Shock and mortality due to S. Choleraesuis infection was defined as hypotension and death occurring within 14 days of the onset of infection. Primary bacteraemia was defined as patients with positive blood cultures for S. Choleraesuis without definite infectious sites. Recurrent infection was defined as a distinct infection event with positive cultures of S. Choleraesuis from blood, body fluid or tissue after resolution of signs and symptoms with complete treatment.
Bacteriology
There were 87 isolates collected from the 81 patients (six isolates were collected from six patients with recurrence). The isolates as were initially identified as S. Choleraesuis by the conventional biochemical tests and the Phoenix System (Becton Dickinson, Sparks, MD, USA) as well as by serotypes determined by the Kauffman and White scheme using somatic and flagellar antigens (Denka Seiken Co. Ltd, Tokyo, Japan) [3]. For susceptibility testing, the standard disk diffusion test was used [7]. The antibiotic disks were purchased from the manufacturer (BBL Microbiology Systems, Cockeysville, MD, USA). Organisms were categorized as susceptible, intermediate, and resistant to the antimicrobial agents tested on the basis of the guidelines provided by the Clinical Laboratory Standard Institute [CLSI, formerly, National Committee for Clinical Laboratory Standards (NCCLS)] [7]. In this study, isolates exhibiting intermediate resistance were classed as resistant. The antibiotics tested and corresponding zone diameters of inhibition used to define resistance were as follows: chloramphenicol (⩽17 mm), ampicillin (⩽16 mm), ciprofloxacin (⩽20 mm), cefotaxime (⩽22 mm), and trimethoprim–sulphamethoxazole (⩽15 mm). Nalidixic acid was not included in the panel of routine disk susceptibility testing.
Statistical analysis
All statistical analyses were completed using version 11 of the SPSS software package (SPSS Inc., Chicago, IL, USA). Continuous variables were expressed as mean±s.d. and compared using Student's t test. The characteristics of patients with and without ciprofloxacin-resistant isolates were compared by χ2 analysis or Fisher's exact test and summarized by proportion in each category. The relation between risk factors and the development of primary bacteraemia and mycotic aneurysm were analysed with the use of logistic regression models. All associated demographic characteristics in Table 1 were analysed separately to first define the relation between S. Choleraesuis primary bacteraemia and mycotic aneurysm. Relevant risk factors were included in a multivariate analysis to calculate mutually adjusted, standardized odds ratios (OR). All P values were two-tailed, and a value of P<0·05 was considered to be statistically significant.
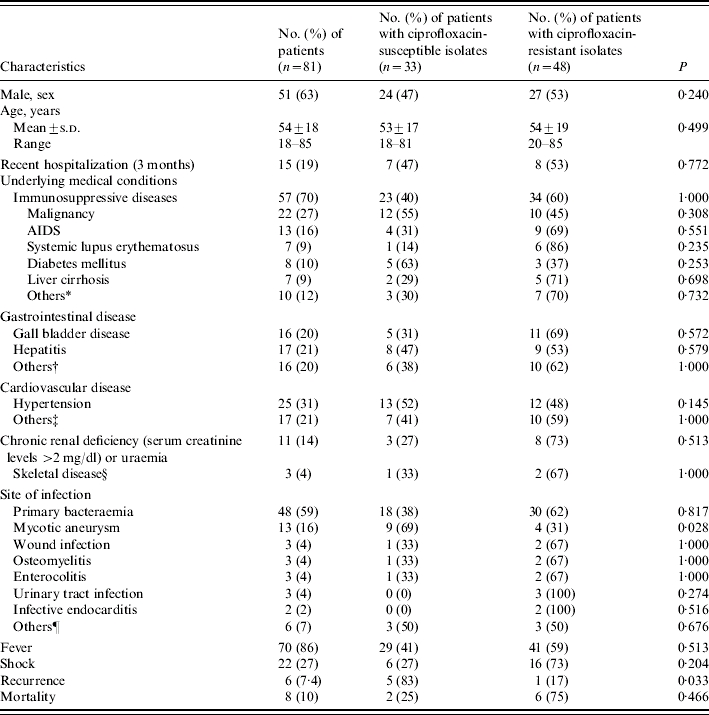
Table 1.
Demographic characteristics and clinical manifestations of patients with S. Choleraesuis bacteraemia: comparison between ciprofloxacin-susceptible and ciprofloxacin-resistant groups
Include diseases receiving immunosuppressive and steroid therapy.
Include gastritis, peptic ulcer, and gastrointestinal bleeding.
Include coronary artery disease, myocardial infarction, rheumatic heart disease, and congestive heart failure.
Include avascular femoral head necrosis and osteoarthritis.
Include cholecyctitis, sinusitis, pneumonia, thyroiditis, and spontaneous bacteria peritonitis.
RESULTS
Disease incidence
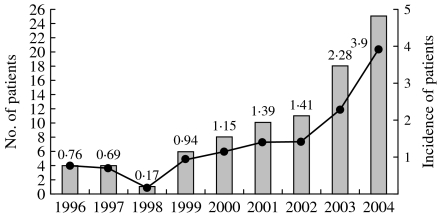
The annual number and incidence (cases per 100 000 discharges) of adult patients with S. Choleraesuis bacteraemia during each year in the study period are shown in Figure 1. The incidence of S. Choleraesuis bacteraemia was 0·76 in 1996 and 0·17 in 1998 and rose gradually from 0·94 in 1999 to 3·9 in 2004.
Fig. 1.
Incidence and number of patients with S. Choleraesuis bacteraemia treated at National Taiwan University Hospital from 1996 to 2004.
 , No. of patients; –●–, incidence (no. of patients per 100 000 discharges).
, No. of patients; –●–, incidence (no. of patients per 100 000 discharges).
Antimicrobial susceptibilities of the isolates
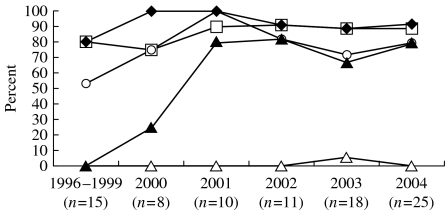
Figure 2 shows the antimicrobial susceptibilities of the 87 isolates obtained during the study period. Resistance to chloramphenicol, ampicillin, and trimethoprim–sulphamethoxazole was found in 92·0, 87·4, and 77·0% isolates respectively. Resistance to ciprofloxacin was found in 59% of isolates. An annual increased rate of ciprofloxacin resistance was noted from 0% prior to 2000, to 25% in 2000, and to 80% in 2004. Most ciprofloxacin-resistant isolates were also resistant to chloramphenicol, ampicillin, and trimethoprim–sulphamethoxazole simultaneously (multidrug resistance). One isolate (1·1%) from a patient who had chronic and recurrent osteomyelitis treated in 2003 was resistant to both cefotaxime and ciprofloxacin.
Fig. 2.
Antimicrobial resistance of 87 isolates S. Choleraesuis recovered from patients treated at National Taiwan University Hospital from 1996 to 2004. Ampicillin (–□–); cotrimoxazole (–○–); chloramphenicol (–◆–); cefotaxime (–△–); ciprofloxacin (–▲–).
Clinical characteristics
Among the 81 patients, 51 (63%) were male and 30 were female. Their ages ranged from 18 to 85 years with a mean of 54 years. The underlying medical conditions, site of infection, and initial presentations of the 81 patients are shown in Table 1. Fifty-seven patients (70%) had immunosuppressive conditions including AIDS, systemic lupus erythematous, malignancy, liver cirrhosis, diabetes mellitus and diseases for which they received immunosuppressive or steroid therapy. Primary bacteraemia (48 patients, 59%) and mycotic aneurysm in aorta (13 patients, 16%) were the two leading sites of infection. Localized infections other than mycotic aneurysm included osteomyelitis in three patients (two patients had femoral head infection and one had vertebral infection), wound infection in three patients, enterocolitis in three patients, and urinary tract infection in three patients. There were six patients with an obvious localized infectious focus which was not proved by positive localized culture of S. Choleraesuis. These foci included cholecystitis, sinusitis, pneumonia, thyroiditis, and spontaneous bacterial peritonitis. Fever was the most common initial presentation and was recorded in 70 patients (86%). Twenty-two patients (27%) developed shock and eight patients (10%) died within 14 days of the onset of symptoms.
S. Choleraesuis isolates with resistance to ciprofloxacin were recovered from 48 (59%) patients. The demographic and underlying conditions of patients with and without ciprofloxacin-resistant isolates are shown in Table 1. There were no significant differences in age, sex, underlying diseases, recent hospitalization, clinical manifestations, or outcomes between these two groups. Patients with mycotic aneurysm were more frequently infected with ciprofloxacin-susceptible isolates rather than ciprofloxacin-resistant isolates (P=0·028). Ciprofloxacin-susceptible isolates tended to cause more recurrent infection than ciprofloxacin-resistant isolates (P=0·038).
Factors associated with S. Choleraesuis primary bacteraemia and mycotic aneurysm
The risk factors significantly associated with the development of primary S. Choleraesuis bacteraemia were hypertension, gall bladder disease and underlying immunosuppressive conditions. In a multivariate logistic regression model that mutually adjusted for the presence of all risk factors, we found that underlying immunosuppressive conditions was an independent risk factor for primary S. Choleraesuis bacteraemia [OR 18·442, 95% confidence interval (CI) 3·845–88·440, P<0·001]. The risk factors significantly associated with mycotic aneurysm were male gender (OR 7·422; 95% CI 1·101–50·021, P=0·039) and hypertension (OR 15·434, 95% CI 2·691–88·515, P=0·002) (Table 2).
Table 2.
Factors associated with S. Choleraesuis primary bacteraemia and mycotic aneurysm, as determined by multivariate analysis


OR, Odds ratio; CI, confidence interval.
Include patients with AIDS, systemic lupus erythematous, malignancy, liver cirrhosis, diabetes mellitus, and other medical conditions necessitating immunosuppressive or steroid therapy.
Recurrence
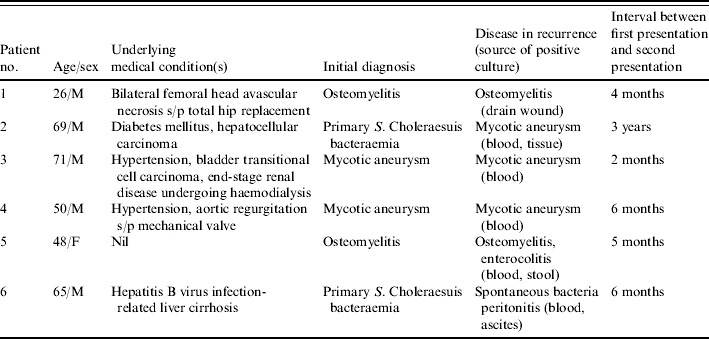
Six patients had recurrent S. Choleraesuis infection (Table 3) with intervals from initial bacteraemia to recurrence ranging from 2 months to 3 years. Among these six patients, two had chronic recurrent osteomyelitis and two had mycotic aneurysm, and these four patients had been treated with either a third-generation cephalosporin or ciprofloxacin without surgery prior to recurrence of bacteraemia. The remaining two patients had primary bacteraemia initially, with recurrence manifesting as mycotic aneurysm in one patient and as spontaneous bacterial peritonitis in the other one. All but one (patient no. 5) of these six patients was infected with ciprofloxacin-susceptible isolates and received either a third-generation cephalosporin or ciprofloxacin in treating their recurrent infection. Patient no. 5 was initially infected with ceftriaxone-susceptible and ciprofloxacin-resistant S. Choleraesuis isolates and then acquired recurrent infection caused by a ceftriaxone-resistant strain.
Table 3.
Summary of clinical features of patients with recurrent S. Choleraesuis infections
DISCUSSION
This study found an increase in the rate of ciprofloxacin-resistant S. Choleraesuis isolates from 0% prior to 2000 to 80% in 2004 and a parallel increase in the incidence of bacteraemic S. Choleraesuis infection. Investigators in Taiwan have demonstrated that most S. Choleraesuis isolates, particularly the ciprofloxacin-resistant isolates, recovered from humans and pigs exhibited the same or similar DNA fingerprints, indicating that human infections were derived from pigs [3, 6]. In Taiwan, quinolone agents are widely used in animal husbandry for growth promotion or therapeutic purposes but are not available over the counter [8]. Infection with isolates that are resistant to antimicrobials may result from prior exposure to these agents [9].
It is suggested that resistant isolates might be somewhat more virulent than susceptible isolates [4, 10]. This assumption might not be true in S. Choleraesuis infection because mycotic aneurysm and recurrent infections described in this study were often caused by ciprofloxacin-susceptible isolates. This study has contradicted this hypothesis.
Various immunosuppressive conditions were noted in 57 patients (70%). This finding is different to two earlier studies from Taiwan [11, 12]. Documented localized infections were found in 33 patients (41%) in this series. The rate of primary S. Choleraesuis bacteraemia was higher in this study (48/81, 59%) than in a previous report from Taiwan [12], which might be due to the higher ratio of various immunosuppressive conditions in the study population.
In this study, multivariate analysis showed that immunosuppressive disease was an independent and the most important risk factor for primary S. Choleraesuis bacteraemia. S. Choleraesuis infections in immunocompromised patients may have a more insidious clinical course. The signs and symptoms, such as localized pain, are somewhat vague and non-specific in immunocompromised patients and this may lead to underestimation of the number of localized infections. Hypertension and gall bladder disease appear to have a protective effect against S. Choleraesuis primary bacteraemia development. This may be due to the fact that patients who had hypertension and gall bladder disease were prone to have localized infection in our study. Two patients in our study who were initially considered to have S. Choleraesuis primary bacteraemia developed recurrent localized infection later after complete treatment. A cautious approach for searching infectious focus is warranted, especially in immunocompromised patients.
In 1962, Sower & Whelan demonstrated that Salmonella was a common pathogen of mycotic aneurysm in patients with pre-existing atherosclerosis [13]. In Taiwan, it appears that Salmonella mycotic aneurysm continues to be more common than in other areas of the world and most of these aneurysms are due to S. Choleraesuis [14–16]. The finding regarding male predominance in patients with mycotic aneurysm is consistent with data reported by Cohen et al. about Salmonella arterial infections [17]. Age-related degenerative disease, such as atherosclerosis, may be a factor that directly predisposes older patients with non-typhoid salmonellosis to mycotic aneurysm [16]. Despite a high rate of Salmonella bacteraemia among patients with various immunocompromised conditions, mycotic aneurysm was rarely found in these patients. In our study, hypertension was the major factor predisposing to S. Choleraesuis mycotic aneurysm.
Recurrence after adequate antimicrobial treatment is a feared clinical scenario in patients with S. Choleraesuis infection. Pre-existing tissue damage or alteration, such as an atherosclerotic plaque, diseased bones or joints, may allow easy seeding by concurrent bacteraemia. The efficacy of antimicrobial therapy in this situation is doubtful. Six out of 81 patients in this study had recurrent S. Choleraesuis infection. Among these six patients, the two who initially presented with mycotic aneurysm were treated with third-generation cephalosporin without surgery and developed recurrent bacteraemia and two had chronic recurrent osteomyelitis. A previous case-series study of mycotic aneurysm from Taiwan by Hsu et al. [18] found that timely surgical intervention and prolonged intravenous antibiotic therapy resulted in excellent outcomes. Fernandez Guerrero et al. [19] also demonstrated that patients with S. enterica mycotic aneurysm treated with both surgery and antibiotics did not have recurrence. These findings indicate that if the diagnosis of mycotic aneurysm is made, surgical resection should follow once adequate antimicrobial therapy has been started. Subsequently, asymptomatic patients should have repeated blood cultures to rule out recurrence.
Data is limited on the management of chronic recurrent Salmonella osteomyelitis. As previously published, the patient (no. 5) had recurrent infection manifesting as bacteraemia, enterocolitis, and osteomyelitis and received different antimicrobial agents (ciprofloxacin, cefepime, aztreonam, and carbapenems) during an 11-month period, which was caused by six closely related isolates (a single strain) exhibiting variable resistance profiles [20]. This suggests that antimicrobial therapy alone does not eradicate Salmonella infection, especially in patients with osteomyelitis.
The emergence of resistance to both ciprofloxacin and ceftriaxone among S. Choleraesuis has been recently reported in Taiwan [21–24]. The growing antimicrobial resistance to newer antimicrobial agents, such as fluoroquinolones and extended spectrum cephalosporins makes therapy more difficult and challenging [21–24]. In this study, 13% of patients with bacteraemia due to ciprofloxacin-resistant isolates died compared with 6% in patients infected with ciprofloxacin-susceptible isolates. However, 10 (30%) ciprofloxacin-susceptible and eight (17%) ciprofloxacin-resistant isolates were associated with invasive diseases (such as mycotic aneurysm, osteomyelitis, and infective endocarditis). Moreover, isolates susceptible to ciprofloxacin tended to cause more recurrent infection than ciprofloxacin-resistant isolates. Further studies should be conducted to elucidate the relationship between invasiveness (virulence) and ciprofloxacin susceptibility of S. Choleraesuis.
Several limitations were found in this retrospective study. First, rates of ciprofloxacin resistance among S. Choleraesuis might be underestimated. Defining resistance to ciprofloxacin for Salmonella species isolated from extra-intestinal sources using the disk diffusion method based on the current NCCLS guideline was inappropriate [7]. A minimum inhibitory concentration (MIC) break-point of ⩾0·12 mg/l, rather than ⩾4 mg/l, for defining resistance to ciprofloxacin is recommended. Aarestrup et al. suggests that testing for susceptibility to nalidixic acid should be performed as a screening tool to predict resistance of ciprofloxacin [25]. Second, several important potential risk factors, including contact history with pigs, pork products or other animals and recent administration of antibiotics (particularly fluoroquinolones), for acquisition of ciprofloxacin-resistant isolates were not analysed due to the lack of information in medical records. The United States Food and Drug Administration has withdrawn the use of enrofloxacin in poultry due to concern about the importance of fluoroquinolone resistance among humans [26]. Further prospective control studies, including environmental surveillance of these resistant pathogens and genetic analysis of isolates from animals, the environment and humans, should be conducted to better define the relationship between poultry contamination and human infections.
In conclusion, immunosuppressive disease is the most important predisposing factor for S. Choleraesuis bacteraemia. Patients frequently have symptomatic bacteraemia as the initial presentation without metastatic localized infection. Despite the advances in diagnostic modalities and treatment, S. Choleraesuis infection remains a life-threatening disease with significant morbidity and mortality. A more aggressive approach to treatment towards S. Choleraesuis infection than is commonly given, with institution of effective antimicrobial therapy followed by surgical intervention in cases with an infectious focus is needed. Such an approach may be essential to eradicate S. Choleraesuis infection initially and prevent later recurrence.
DECLARATION OF INTEREST
None.
REFERENCES
- 1.Chiu CH, Su LH, Chu C. Salmonella enterica serotype Choleraesuis: epidemiology, pathogenesis, clinical disease, and treatment. Clinical Microbiology Reviews. 2004;17:311–322. doi: 10.1128/CMR.17.2.311-322.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bopp CA, Murray PR, Baron EJ Manual of Clinical Microbiology. 8th edn. Washington: American Society for Microbiology; 2003. Escherichia, Shigella, and Salmonella; pp. 654–671. : pp. [Google Scholar]
- 3.Hsueh PR et al. Ciprofloxacin-resistant Salmonella enterica Typhimurium and Choleraesuis from pigs to humans, Taiwan. Emerging Infectious Diseases. 2004;10:60–68. doi: 10.3201/eid1001.030171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Su LH et al. Antimicrobial resistance in nontyphoid Salmonella serotypes: a global challenge. Clinical Infectious Diseases. 2004;39:546–551. doi: 10.1086/422726. [DOI] [PubMed] [Google Scholar]
- 5.Chiu CH et al. Fluoroquinolone resistance in Salmonella enterica serotype Choleraesuis, Taiwan, 2000–2003. Emerging Infectious Diseases. 2004;10:1674–1676. doi: 10.3201/eid1009.030596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chiu CH et al. The emergence in Taiwan of fluoroquinolone resistance in Salmonella enterica serotype Choleraesuis. New England Journal of Medicine. 2002;346:413–419. doi: 10.1056/NEJMoa012261. [DOI] [PubMed] [Google Scholar]
- 7.National Committee for Clinical Laboratory Standards Wayne, PA: NCCLS; 2005. . Performance standards for antimicrobial susceptibility testing: fifteen informational supplement. M100-S15. [Google Scholar]
- 8.McDonald LC et al. The use of antibiotics critical to human medicine in food-producing animals in Taiwan. Journal of Microbiology, Immunology and Infection. 2001;34:97–102. [PubMed] [Google Scholar]
- 9.Glynn MK et al. Prior antimicrobial agent use increases the risk of sporadic infections with multidrug-resistant Salmonella enterica serotype Typhimurium: a FoodNet case-control study, 1996–1997. Clinical Infectious Diseases. 2004;38:S227–S236. doi: 10.1086/381591. [DOI] [PubMed] [Google Scholar]
- 10.Travers K, Barza M. Morbidity of infections caused by antimicrobial-resistant bacteria. Clinical Infectious Diseases. 2002;34:S131–S134. doi: 10.1086/340251. (Suppl. 3): [DOI] [PubMed] [Google Scholar]
- 11.Chen YH et al. Salmonella choleraesuis bacteremia in southern Taiwan. Kaohsiung Journal of Medical Sciences. 1999;15:202–208. [PubMed] [Google Scholar]
- 12.Chiu S, Chiu CH, Lin TY. Salmonella enterica serotype Choleraesuis infection in a medical center in northern Taiwan. Journal of Microbiology, Immunology and Infection. 2004;37:99–102. [PubMed] [Google Scholar]
- 13.Sower ND, Whelan TJ., Jr Suppurative arteritis due to Salmonella. Surgery. 1962;52:851–859. [PubMed] [Google Scholar]
- 14.Wang JH et al. Mycotic aneurysm due to non-typhi Salmonella: report of 16 cases. Clinical Infectious Diseases. 1996;23:743–747. doi: 10.1093/clinids/23.4.743. [DOI] [PubMed] [Google Scholar]
- 15.Chan P et al. Salmonellosis and mycotic aneurysm of the aorta Journal of Infection. 1995;30:129–133. doi: 10.1016/s0163-4453(95)80007-7. . A report of 10 cases. [DOI] [PubMed] [Google Scholar]
- 16.Hsu RB et al. Risk factors for primary bacteremia and endovascular infection in patients without acquired immunodeficiency syndrome who have nontyphoid salmonellosis. Clinical Infectious Diseases. 2003;36:829–834. doi: 10.1086/367932. [DOI] [PubMed] [Google Scholar]
- 17.Cohen JI, Bartlett JA, Corey GR. Extra-intestinal manifestations of Salmonella infections. Medicine. 1987;66:349–388. doi: 10.1097/00005792-198709000-00003. [DOI] [PubMed] [Google Scholar]
- 18.Hsu RB et al. Management of aortic aneurysm infected with Salmonella. British Journal of Surgery. 2003;90:1080–1084. doi: 10.1002/bjs.4170. [DOI] [PubMed] [Google Scholar]
- 19.Fernandez Guerrero ML et al. The spectrum of cardiovascular infections due to Salmonella enterica: a review of clinical features and factors determining outcome. Medicine. 2004;83:123–138. doi: 10.1097/01.md.0000125652.75260.cf. [DOI] [PubMed] [Google Scholar]
- 20.Sun HY et al. Occurrence of ceftriaxone resistance in ciprofloxacin-resistant Salmonella enterica serotype Choleraesuis isolates causing recurrent infection. Clinical Infectious Diseases. 2005;40:208–209. doi: 10.1086/426695. [DOI] [PubMed] [Google Scholar]
- 21.Yan JJ et al. Emergence of ceftriaxone-resistant Salmonella isolates and rapid spread of plasmid-encoded CMY-2-like cephalosporinase, Taiwan. Emerging Infectious Diseases. 2003;9:323–328. doi: 10.3201/eid0903.010410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jean SS et al. Recurrent infections caused by cefotaxime- and ciprofloxacin-resistant Salmonella enterica seropype Choleraesuis treated successfully with imipenem. Journal of Infection. 2005 doi: 10.1016/j.jinf.2004.12.011. (in press). [DOI] [PubMed] [Google Scholar]
- 23.Ko WC et al. A new therapeutic challenge for old pathogens: community-acquired invasive infections caused by ceftriaxone- and ciprofloxacin-resistant Salmonella enterica serotype Choleraesuis. Clinical Infectious Diseases. 2005;40:315–318. doi: 10.1086/426593. [DOI] [PubMed] [Google Scholar]
- 24.Chiu CH et al. Isolation of Salmonella enterica serotype choleraesuis resistant to ceftriaxone and ciprofloxacin. Lancet. 2004;363:1285–1286. doi: 10.1016/S0140-6736(04)16003-0. [DOI] [PubMed] [Google Scholar]
- 25.Aarestrup FM et al. Is it time to change fluoroquinolone breakpoints for Salmonella spp. Antimicrobial Agents and Chemotherapy. 2003;47:827–829. doi: 10.1128/AAC.47.2.827-829.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.U.S. Food and Drug Administration . Withdrawal of approval of the new animal drug application for enrofloxacin in poultry. 2000N-1571. Fed Reg.