SUMMARY
Rotaviruses are a major cause of hospitalizations for acute gastroenteritis in developed countries. This review shows the burden of rotavirus disease in <5-year-old children in Europe. An estimated 72 000–77 000 hospitalizations for community-acquired rotavirus disease occur annually in the 23 million under-fives living in the European Union (EU-25), with a median cost of €1417 per case. Annual hospitalization incidence rates range from 0·3 to 11·9/1000 children <5 years old (median 3/1000). The median proportion of hospital-acquired rotavirus disease among all cases of hospitalization for rotavirus disease is estimated to be 21%. Countries of the EU-25 require information on the burden of rotavirus disease to support introduction of rotavirus vaccines. Data on cases treated at home, medical visits, and emergency wards as well as rotavirus-associated deaths are limited. To fully evaluate the impact and effectiveness of rotavirus vaccination programmes in Europe, additional epidemiological studies will be critical and desirable.
INTRODUCTION
Rotaviruses are members of the Reoviridae [1, 2]. They are a major cause of acute gastroenteritis in infants and young children worldwide, are transmitted faecal-orally and are highly infectious. Diarrhoea and vomiting may lead to serious dehydration and death if untreated. Treatment is mainly by oral or intravenous rehydration [3]. Repeated natural rotavirus infections build up protection against disease [4]. The epidemiology is complex with co-circulation of unpredictable changes of different rotaviruses types in different regions at different times of the year [1, 2]. Rotavirus disease peaks between 6 and 24 months of age, and most clinically significant gastroenteritis episodes, including those requiring hospital admission, occur before the age of 5 years.
A recent update of the global burden of rotavirus disease estimated 111 million episodes at home, 25 million outpatient visits, 2 million in-patient visits and over 600 000 deaths [5, 6]. Most of this disease burden is in developing countries, reflecting the large number of children aged <5 years old, as well as a higher case-fatality rate due to underlying risk factors such as malnutrition, concomitant infections and limited access to health-care. Nevertheless the relative burden of rotavirus disease (measured as incidence rates) is comparable in both developing and developed countries [5]. This indicates that hygienic measures alone are not enough. Therefore there is a need for effective prevention strategies to reduce rotavirus morbidity.
Severe rotavirus disease is largely preventable by vaccination with live attenuated oral vaccines [7]. This was demonstrated with the use of a rhesus rotavirus-based tetravalent human reassortant vaccine (RRV-TV) [8–10], and two new rotavirus vaccines being studied [11, 12] are likely to be introduced soon. Rotavirus vaccination will be of interest to developed countries for a number of reasons of which a major one is to achieve a reduction of the large, and costly, burden of hospitalizations for severe rotavirus disease. Policy makers require information on the burden of rotavirus disease and cost-effectiveness of the vaccine to help in their decision-making processes.
This review summarizes the available data for Europe on the burden of rotavirus disease in children <5 years old on hospitals, emergency-room visits, and primary care. Gaps in the information on the burden of rotavirus disease and mortality in Europe, as well as associated costs, were identified.
METHODS
The references selected for this review were identified by Medline searches. To identify papers on the burden of rotavirus disease the following broad search strategy was defined: ‘rotavirus’ (text word) combined with ‘country name’ (text word or in author's affiliation) and combined with ‘epidemiology’ (medical subheading). The search was limited to the period 1994–2005 and to studies in humans. This search was performed for every European country of the WHO European region separately (North, West, South, Central and East European countries including all former USSR countries and Turkey) and yielded 161 papers. Further inclusion criteria were the languages Dutch, English, French, German, Italian and Spanish; studies containing data for children <5 years old; the observation period being a whole year(s) period (except for data on nosocomial infection); and limited to group A rotavirus infections. Finally, 59 papers containing data relevant to the objectives of this paper were identified. Some additional studies mentioned in the references of the papers retrieved by the initial search were considered for inclusion, including key papers published before 1994.
Although the Medline search strategy was performed for all countries of the WHO European region, data were mostly available for the European Union (EU-25) countries. Therefore, estimates for the absolute disease burden were limited to the EU-25 region. The rates for medical visits and hospitalizations are population-based.
RESULTS
The impact of rotavirus disease at a country level was split in six categories: (1) cases treated at home (without a visit to physician), (2) disease treated at the level of primary health care (medical visits), (3) disease seen and treated in hospital emergency wards, (4) community-acquired disease requiring hospitalization with (5) hospital-acquired (nosocomial) infections regarded as a separate entity and (6) mortality.
Cases treated at home
It has been estimated that by the age of 5 years nearly every child will have had one or more rotavirus infections [5], but information on the proportion of symptomatic disease is limited. In a prospective cohort of 336 Finnish infants followed from birth to 2·5 years of age 1 in 7 infants had a symptomatic episode of rotavirus disease [13]. In another cohort of Finnish infants followed prospectively in a vaccine clinical trial (the placebo group of a RRV-TV trial) [9], 188 symptomatic cases of rotavirus infection occurred among 1145 infants followed for 1 year. This corresponds to about one symptomatic case in six infants [9]. Of these, only 28% were treated at home. To our knowledge, no other reports on rotavirus cases treated at home are available.
Primary health care: general practitioner, family paediatrician, and outpatient clinic
In the Finnish vaccine trial 86 of 1145 infants in the placebo group of the RRV-TV vaccine trial visited a physician for rotavirus disease [14], corresponding to 7·5/100 infants per year. Prospective observational studies in paediatricians' offices in Austria, Switzerland, and Germany gave annual incidence rates of 0·84, 1·8, and 4·1/100 children aged <5 years old respectively [15–18]. In The Netherlands, 0·76/100 children aged <1 year old and 0·46/100 children aged 1–4 years old visited the GP for rotavirus disease each year, telephone consultations were excluded [19, 20].
Emergency wards
In the Paris area (France) rotavirus infections accounted for 2–2·4% of all paediatric consultations at the emergency department [21]. In the Basque country (Spain), the incidence rate of rotavirus disease treated at the emergency ward was 2·2 cases/100 children aged <4 years old [22]. A similar incidence rate (2·6/100) was found in infants in the placebo group of the Finnish RRV-TV vaccine trial [9]. In this trial 1·8 visits were made to the emergency department for every hospitalization for rotavirus disease [9]. This ratio is likely to be lower when the emergency wards are equipped with facilities to provide 24-h intravenous fluid replacement. A study in Italy showed that 10 cases could be treated in the emergency ward for every case admitted to hospital (C. Giaquinto, unpublished observations).
Hospitalizations
In the EU-25 region 6–11% of all hospitalizations of children aged <5 years old are caused by acute gastroenteritis [23–27], and a median of 40% (range 14–54%) of acute gastroenteritis hospitalizations is attributable to rotavirus infection [17, 25, 26, 28–40]. The reported percentage of rotavirus disease in acute gastroenteritis hospitalizations clearly depends on study design, coverage and representativeness of laboratory surveillance systems, as well as the year(s) and geographical area(s) included in the study.
An overview of the incidence rates of hospitalization of children aged <5 years old due to community-acquired rotavirus disease in Europe (Table 1 estimates a median incidence rate of 3/1000 children per year (range 0·3–11·9/1000), median cumulative incidence rate of 1 in 67 children hospitalized because of rotavirus gastroenteritis before the age of 5 years, and 72 000–77 000 hospitalizations per annum of <5-year-old children for rotavirus disease in the EU-25 region.
Table 1.
Population-based hospitalization incidence rates for community-acquired rotavirus (RV) disease in European countries
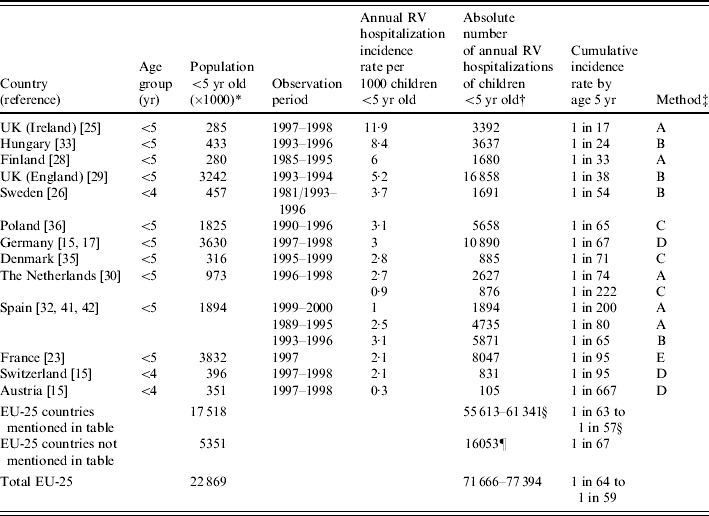
Estimates for 2005. UN Department of Economic and Social Affairs. Population Division. World Population Prospects: The 2002 Revision Population Database. United Nations, New York, 2004 (http://esa.un.org/unpp/index.asp?panel=1).
Data for children <4 years old have been applied to the population of children <5 years old.
A, direct or indirect estimate (logistic regression) based on national hospital admission data for acute gastroenteritis and laboratory data for rotavirus infection; B, same method as A, but based on regional data; C, hospital-based studies testing all or a sample of acute gastroenteritis admissions; D, prospective study among representative sample of paediatric offices and subsequent hospital admissions (laboratory-confirmed cases); E, direct estimate based on the International Classification of Diseases and Causes of Death (9th revision) code for rotavirus disease in national hospital discharge data.
The absolute numbers of the individual EU-25 countries in the table (thus excluding Switzerland) have been summed. A range is given, because for some countries more than one estimate of the absolute number of RV cases was available. The cumulative incidence is based on the estimated absolute number of cases and the population size.
For EU-25 countries for which no data were available from the literature, the absolute number of cases has been estimated from the median hospitalization incidence rate for EU-25 countries in the table (median 3·0/1000). The cumulative incidence is based on the estimated absolute number of cases and the population size.
In the Finnish RRV-TV vaccine trial 13 children were hospitalised for rotavirus disease among 1145 infants [9] in the placebo group, corresponding to 11·4/1000 infants per year – comparable to the high end rate seen in the British Isles (Ireland) [25].
The average length of hospital-stay ranged from 2 to 9·5 days (median 4·8 days) [17, 23, 27–29, 33, 34, 36, 41–43]. The longest durations were found in Central Europe with 8·3 days for infants in Hungary, and 9·5 days for children <5 years old in Poland.
Depending on the studies and countries the hospitalization incidence peaked in children aged 6–11 or 12–23 months [22, 23, 28, 31, 41, 42]. Around 50–60% of the cases occurred in children <1 year old [26, 34, 44], and 60–80% in children <2 years old [28, 30, 34, 36, 41]. In Europe, as in the rest of the world, the risk of being hospitalized for rotavirus disease was highest among children aged <2 years old.
The related direct medical costs per hospitalized case of community-acquired rotavirus disease ranged from €691 in Poland to €1773 in Spain (median: €1417 per case of rotavirus disease, Austria excluded) (Table 2).
Table 2.
Direct medical costs per case hospitalized with community-acquired rotavirus disease in European countries
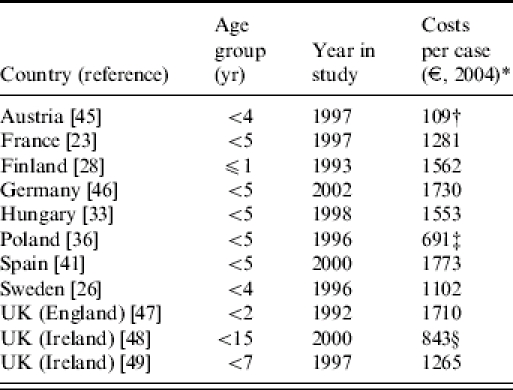
Cost estimate for 2004. Price levels in study corrected for inflation. Cost calculations differ for countries using the cost of bed-days alone (Ireland [49]) or including diagnostics and medication (Austria, France, Hungary, Poland, England, Ireland [48], Spain (?)), as well as doctor's wages (Finland, Germany (?)) or using the reimbursement fee the hospital obtains per case (irrespective of length of stay) (Sweden). (?) Cost calculation not completely clear from the paper.
Calculation based on two cases only.
Prices are considered as ‘severely underestimated’, and ‘expected to rise steeply to EU levels’ [36].
Calculation based on median bed-days and median investigations, interventions and medication. When the calculation was based on actual costs, the mean cost per case was €1409.
Hospital-acquired rotavirus infections
The burden of hospital acquired (nosocomial) rotavirus infections is summarized in Table 3 and described as attack rates (percentage of hospitalized children developing rotavirus disease) and incidence densities (rotavirus disease cases/1000 bed days), respectively. Data were available for seven countries. Of the children aged <5 years old 2·5–11·8% developed rotavirus disease [50–59] during hospitalization (including two studies using onsets at 48 and 72 h after admission). The median proportion of nosocomial rotavirus infection among all rotavirus-associated hospitalizations (community- and hospital-acquired) was estimated to be 21% (range 5–51%) in European countries [21, 22, 27, 30, 33, 36, 47, 60]. About half of all nosocomial rotavirus infections prolonged the length of stay [15, 36] by a number of excess hospital days ranging from 2 to 7 days (median 4·3 days) [15, 36, 48, 50–53, 58].
Table 3.
Nosocomial attack rates and incidence density rates for rotavirus (RV) disease in European countries
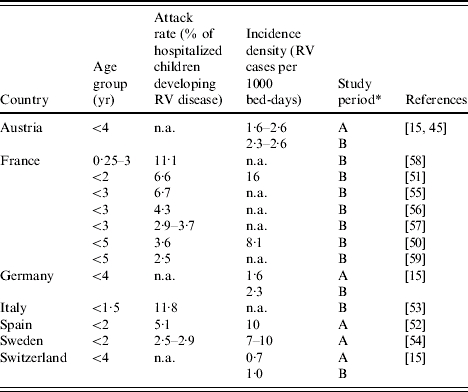
A, whole year(s) period; B, rotavirus season.
n.a., Not available.
Mortality
Estimates on mortality from rotavirus infection could only be made for a few North and West European countries. In Finland one death from rotavirus disease was reported every 2–5 years [28], corresponding to an annual mortality rate of 0·7–1·8 rotavirus deaths per million children aged <5 years old. In France, an estimated 2–6 children aged <5 years old die from rotavirus disease each year [23], equivalent to an annual mortality rate of 0·5–1·6 rotavirus deaths per million children aged <5 years old. In a German children's hospital a mortality rate of 1/1000 rotavirus hospitalizations was found [27]. Using the hospitalization incidence for rotavirus disease for Germany of 3/1000 children aged <5 years old [15], an annual mortality rate of 3 per million children aged <5 years old is estimated. In Ireland, 1 rotavirus death occurred in 11 907 hospitalizations for diarrhoea. The hospitalization-incidence rate for diarrhoea for children aged <5 years old was 24/1000 [25], leading to an estimate of 1·9 rotavirus deaths per million children <5 years old per annum. In England and Wales, 18 deaths from infectious intestinal disease occurred among children aged <5 years old. Laboratory data showed that 39% of the infectious intestinal diseases were caused by rotavirus infection, leading to seven rotavirus deaths in young children per year [61] and an annual mortality rate of 2·3 deaths per million children aged <5 years old. In summary, the mortality rate in North and West European countries ranged from 0·5 to 3 deaths per million children aged <5 years old per year.
DISCUSSION
This review shows that in the EU-25 region 40% (median) of the hospitalizations for acute gastroenteritis in children aged <5 years old were attributable to rotavirus disease, comparable to Asia (45%) [62], and close to the upper limit of 26–49% recently estimated for the United States [63]. Based on the country-specific hospitalization incidence rates for rotavirus disease, this review estimates a minimum of 72 000–77 000 hospitalizations per annum among children aged <5 years old with community-acquired rotavirus disease in the EU-25 region (Table 1). Nosocomial rotavirus disease adds to the hospital burden of disease. An estimated median of 21% of all (community-acquired and nosocomial) rotavirus-associated hospital cases in the EU were nosocomial, adding about 20 000 nosocomial cases of rotavirus disease to the burden of hospitals. This estimate is in line with another recent study which estimated a median of 27% of all rotavirus-associated hospital cases in developed countries to be hospital-acquired [64].
The differences in hospitalization rates may reflect genuine geographical differences with a different epidemiological pattern of disease in North and East Europe compared to West and Central Europe. While the available data do not allow for a proper comparison of the incidence rates between different European countries, they nevertheless suggest that true regional differences exist within Europe. The differences may also reflect genuine differences in disease severity between countries. The prospective studies in Austria, Germany and Switzerland, however, did not reveal an association between disease severity measured with the Vesikari score [65] and hospitalization rate or primary-care visits [15], suggesting that factors other than disease severity affect the differences in hospitalization rates as well. The differences in burden of rotavirus disease between countries may also be due to differences in study design, sample size, age groups included in the studies, differences in health-seeking behaviour, access to health care, and case-disease management.
The Finnish clinical trial data [14] showed fairly high incidence of rotavirus disease in primary and hospital care compared to most observational studies from other countries. The study design of observational studies and clinical trials is very different. Clinical trials have a strictly controlled study design with a very stringent follow-up of cases. Thus, the experimental setting of a trial is likely to lead to higher estimates than the non-experimental setting of the studies based on hospital data as described in Table 1.
In the observational studies a high hospitalization incidence rate was found for Ireland and a low rate for Austria. The authors of the Irish study suggested that the quality of their data sources might be better than in other countries, or there might be a greater tendency to hospitalize children with diarrhoea than to treat them in the emergency room or as outpatients, but there are no data to support this hypothesis [25]. A low incidence rate for Austria was estimated from a population-based prospective study with about 3500 children-years under observation, comparable to the size of the Swiss cohort in the same study [15]. The number of outpatient rotavirus cases was low in Austria, and especially the percentage of outpatient cases that needed hospitalization was very low (4%) in Austria compared to Switzerland (12%), leading to a very low hospitalization incidence rate [15]. However, the differences between Austria and Switzerland were not significantly different [15].
Most studies estimated the incidence rates from either national or regional hospital discharge data on acute gastroenteritis and from the detection rate of rotavirus infection confirmed by laboratory data. It has been shown that this method, preferably using a combination of routine laboratory data and a systematic surveillance programme, yields accurate estimates of rotavirus hospitalizations [63]. Studies based on national hospital discharge data using the specific ICD-9 code for rotavirus disease only (e.g. France [23]) grossly underestimate the real disease burden [63, 66].
For nosocomial rotavirus disease different case definitions were used in published studies, ranging from infection occurring 48–72 h after admission, and the studies were often performed during the rotavirus season only.
The use of Medline as a source of information might have been a limitation as studies in local languages and contexts were not reported. Therefore, little data on Central and East European countries could be presented.
The median cost of €1417 for a hospital stay for community-acquired rotavirus disease is a rough estimate for countries of the EU-25. The figures presented are based on different cost calculations using the cost of bed-days alone or including diagnostics and medication, or using the reimbursement fee the hospital obtains per case (irrespective of length of stay). European data on the cost structure of rotavirus disease show that hospital stay is a main cost driver, but that non-medical costs are not negligible. In the Finnish vaccine trial, hospitalizations accounted for 75% of the medical costs [14, 28]. The total medical costs accounted for 88% and the non-medical costs (including travel and production loss of caregivers) 12% of the total costs of rotavirus disease. For Germany, it was estimated that hospital stay accounted for 51% of the costs associated with rotavirus disease (including nosocomial cases), outpatient visits for 27% and days of work lost by the mother for another 21% [46]. In Austria the productivity loss of the caregiver was estimated to be only 6·6% of the total costs [45]. The costs and cost structure associated with rotavirus disease differ between European countries, depending on the structure of health services and social system (e.g. the period of maternity leave, participation of mothers in employment, etc.) in the country.
There is a need for effective prevention strategies to reduce rotavirus morbidity. Hygienic measures alone do not seem to be enough as incidences of severe disease in developing and developed countries are comparable. In addition, nosocomial transmission is difficult to control with preventive methods of hygiene and isolation due to the resistance of the virus to the environment, and other possible routes of transmission beside the faecal–oral route [57, 64]. During the rotavirus season, the epidemic peak of rotavirus disease leads to a substantial disruption of the hospital care system. The hospitalization rate can increase 1·8- to 2·5-fold compared to a whole year's period [28, 34, 41]. Vaccination could overcome the shortcomings of other prevention strategies. The most tangible primary impact of a rotavirus vaccination programme in Europe would be a reduction of severe rotavirus disease requiring hospitalization as well as nosocomial rotavirus infection. New rotavirus vaccines are likely to become widely available in the near future. In order to evaluate the impact and effectiveness of a vaccination programme, data on the burden of rotavirus disease and its associated costs are required. The available data on the burden of rotavirus disease show great variability in study design and case definition. Data on cases treated at home, medical visits and emergency wards for rotavirus diarrhoea are limited, as well as on the associated costs, and the true number of rotavirus-associated deaths is not known. Standardized surveillance studies addressing the rotavirus disease burden and its determinants for different settings of health care as well as economic studies on medical and non-medical costs are needed.
ACKNOWLEDGEMENTS
The authors thank Dr Montse Sorianoabarro, Dr François Meurice and Dr Beatrice De Vos from GSK Biologicals, Belgium for their comments on the paper.
APPENDIX. Writing Committee of PROTECT Advisory Board
Krisztián Banyai (Hungary); Ulrich Desselberger (United Kingdom); Elisabetta Franco (Italy); Carlo Giaquinto (Chairman) (Italy); Emmanuel Grimprel (France); Hans-Iko Huppertz (Germany); François Meurice (GSK Biologicals, Belgium); Zsofia Mezsner (Hungary); Jacek Mrukowicz (Poland); Carlos Rodrigo (Spain); Montse Soriano-Gabarro (GSK Biologicals, Belgium); Vladimir Tatochenko (Russia); Timo Vesikari (Finland); Beatrice De Vos (GSK Biologicals, Belgium); Judith Wolleswinkel-van den Bosch (Pallas Health Research, Netherlands).
DECLARATION OF INTEREST
The activities of The Pediatric ROTavirus European CommitTee (PROTECT) are sponsored by GlaxoSmithKline Biologicals.
REFERENCES
- 1.Kapikian AZ, Hoshino Y, Chanock RM., Knipe DM, Howley PM. Fields Virology. 4th edn. Philadelphia, PA: Lippincott Williams & Wilkins; 2001. Rotaviruses; pp. 1787–1833. : pp. [Google Scholar]
- 2.Estes MK., Knipe DM, Howley PM. Fields Virology. 4th edn. Philadelphia, PA: Lippincott Williams & Wilkins; 2001. Rotaviruses and their replication; pp. 1747–1785. : pp. [Google Scholar]
- 3.Bass D., Desselberger U, Gray J. Viral Gastroenteritis. Amsterdam: Elsevier Science; 2003. Treatment of viral gastroenteritis; pp. 93–104. : pp. [Google Scholar]
- 4.Velazquez FR et al. Rotavirus infections in infants as protection against subsequent infections. New England Journal of Medicine. 1996;335:1022–1028. doi: 10.1056/NEJM199610033351404. [DOI] [PubMed] [Google Scholar]
- 5.Parashar UD et al. Global illness and deaths caused by rotavirus disease in children. Emerging Infectious Diseases. 2003;9:565–572. doi: 10.3201/eid0905.020562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Parashar UD. Mexico City, Mexico: Rotavirus and rotavirus vaccines. Proceedings of the 6th International Rotavirus Symposium, , 7–9 July 2004. [Google Scholar]
- 7.Centers for Disease Control and Prevention . Rotavirus vaccine for the prevention of rotavirus gastroenteritis among children: recommendations of the Advisory Committee on Immunization Practices (ACIP). Morbidity and Mortality Weekly Report199948 (No. RR-2).
- 8.Rennels MB et al. Safety and efficacy of high-dose rhesus-human reassortant rotavirus vaccines – report of the National Multicenter Trial. United States Rotavirus Vaccine Efficacy Group. Pediatrics. 1996;97:7–13. [PubMed] [Google Scholar]
- 9.Joensuu J, Koskenniemi E, Vesikari T. Randomised placebo-controlled trial of rhesus-human reassortant rotavirus vaccine for prevention of severe rotavirus gastroenteritis. Lancet. 1997;350:1205–1209. doi: 10.1016/S0140-6736(97)05118-0. [DOI] [PubMed] [Google Scholar]
- 10.Perez-Schael I et al. Efficacy of the rhesus rotavirus-based quadrivalent vaccine in infants and young children in Venezuela. New England Journal of Medicine. 1997;337:1181–1187. doi: 10.1056/NEJM199710233371701. [DOI] [PubMed] [Google Scholar]
- 11.De Vos B et al. A rotavirus vaccine for prophylaxis of infants against rotavirus gastroenteritis. Pediatric Infectious Disease Journal. 2004;23:S179–S182. doi: 10.1097/01.inf.0000142370.16514.4a. (Suppl 10): [DOI] [PubMed] [Google Scholar]
- 12.Vesikari Tet al. The effect of dose and composition of a pentavalent rotavirus reassortant vaccine (RotaTeqTM) upon efficacy and immunogenicity in healthy infants. In 40th Annual Meeting of the Infectious Disease Society of America. Chicago, USA: the Infectious Disease Society of America, 2002 [Abstract 35].
- 13.Ruuska T, Vesikari T. A prospective study of acute diarrhoea in Finnish children from birth to 2·5 years of age: clinical severity, etiology, and risk factors. Acta Paediatrica Scandinavica. 1991;80:500–507. doi: 10.1111/j.1651-2227.1991.tb11893.x. [DOI] [PubMed] [Google Scholar]
- 14.Takala AK et al. Economic evaluation of rotavirus vaccinations in Finland: randomized, double-blind, placebo-controlled trial of tetravalent rhesus rotavirus vaccine. Clinical Infectious Diseases. 1998;27:272–282. doi: 10.1086/514650. [DOI] [PubMed] [Google Scholar]
- 15.Frühwirth M et al. International variation in disease burden of rotavirus gastroenteritis in children with community- and nosocomially acquired infection. Pediatric Infectious Disease Journal. 2001;20:784–791. doi: 10.1097/00006454-200108000-00013. [DOI] [PubMed] [Google Scholar]
- 16.Frühwirth M et al. A prospective evaluation of commuity acquired gastroenteritis in paediatric practices: impact and disease burden of rotavirus infection. Archives of Diseases in Childhood. 2001;84:393–397. doi: 10.1136/adc.84.5.393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ehlken B et al. Prospective population-based study on rotavirus disease in Germany. Acta Paediatrica. 2002;91:769–775. doi: 10.1080/08035250213227. [DOI] [PubMed] [Google Scholar]
- 18.Laubereau B et al. Rotavirus gastroenteritis in infants and children. Results of a prospective study in the area of Geneva and Basel 1997/1998 (RoMoS). RoMoS Study Group [in German] Schweizerische Medizinische Wochenschrift. 1999;129:1822–1830. [PubMed] [Google Scholar]
- 19.De Wit MAS et al. Gastroenteritis in sentinel general practices, the Netherlands. Emerging Infectious Diseases. 2001;7:82–91. doi: 10.3201/eid0701.010113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.De Wit MAS et al. Etiology of gastroenteritis in sentinel general practices in The Netherlands. Clinical Infectious Diseases. 2001;33:280–287. doi: 10.1086/321875. [DOI] [PubMed] [Google Scholar]
- 21.Grimprel E et al. Acute diarrhoea and rotavirus infection in the child: assessment of data from emergency care and the microbiology laboratory of the Armand-Trousseau (Paris) Hospital between 1988 and 2001 [in French] Archives de Pédiatrie. 2001;8:1318–1324. doi: 10.1016/s0929-693x(01)00652-2. [DOI] [PubMed] [Google Scholar]
- 22.Cilla G et al. Incidence, seasonality and serotypes of rotavirus in Gipuzkoa (Basque Country), Spain. A 14-year study. Epidemiology and Infection. 2000;125:677–683. doi: 10.1017/s0950268800004842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fourquet F et al. Acute gastro-enteritis in children in France: estimates of disease burden through national hospital discharge data. Archives de Pédiatrie. 2003;10:861–868. doi: 10.1016/s0929-693x(03)00459-7. [DOI] [PubMed] [Google Scholar]
- 24.Desenclos JC et al. Diarrhoea-related morbidity and rotavirus infection in France. Acta Paediatrica. 1999;426:42–47. doi: 10.1111/j.1651-2227.1999.tb14325.x. [DOI] [PubMed] [Google Scholar]
- 25.Lynch M et al. Rotavirus in Ireland: national estimates of disease burden, 1997 to 1998. Pediatric Infectious Disease Journal. 2001;20:693–698. doi: 10.1097/00006454-200107000-00010. [DOI] [PubMed] [Google Scholar]
- 26.Johansen K et al. Incidence and estimates of the disease burden of rotavirus in Sweden. Acta Paediatrica. 1999;426:20–23. doi: 10.1111/j.1651-2227.1999.tb14321.x. [DOI] [PubMed] [Google Scholar]
- 27.Berner R et al. Occurrence and impact of community-acquired and nosocomial rotavirus infections – a hospital-based study over 10 y. Acta Paediatrica. 1999;426:48–52. doi: 10.1111/j.1651-2227.1999.tb14326.x. [DOI] [PubMed] [Google Scholar]
- 28.Vesikari T, Rautanen T, Von Bonsdorff C-H. Rotavirus gastroenteritis in Finland: burden of disease and epidemiological features. Acta Paediatrica. 1999;426:24–30. doi: 10.1111/j.1651-2227.1999.tb14322.x. [DOI] [PubMed] [Google Scholar]
- 29.Ryan MJ et al. Hospital admissions attributable to rotavirus infection in England and Wales. Journal of Infectious Diseases. 1996;174:S12–S18. doi: 10.1093/infdis/174.supplement_1.s12. [DOI] [PubMed] [Google Scholar]
- 30.De Wit MAS et al. Hospital admissions for rotavirus infection in the Netherlands. Clinical Infectious Diseases. 2000;31:698–704. doi: 10.1086/314025. [DOI] [PubMed] [Google Scholar]
- 31.Ruggeri FM, Declich S. Rotavirus infection among children with diarrhoea in Italy. Acta Paediatrica. 1999;426:66–71. doi: 10.1111/j.1651-2227.1999.tb14329.x. [DOI] [PubMed] [Google Scholar]
- 32.Visser LE et al. Impact of rotavirus disease in Spain: an estimate of hospital admissions due to rotavirus. Acta Paediatrica. 1999;426:72–76. doi: 10.1111/j.1651-2227.1999.tb14330.x. [DOI] [PubMed] [Google Scholar]
- 33.Szücs G et al. Burden of human rotavirus-associated hospitalizations in three geographical regions of Hungary. Acta Paediatrica. 1999;426:61–65. doi: 10.1111/j.1651-2227.1999.tb14328.x. [DOI] [PubMed] [Google Scholar]
- 34.Kurugöl Z et al. Rotavirus gastroenteritis among children under five years of age in Izmir, Turkey. Turkish Journal of Pediatrics. 2003;45:290–294. [PubMed] [Google Scholar]
- 35.Fischer TK. Incidence of hospitalizations due to rotavirus gastroenteritis in Denmark. Acta Paediatrica. 2001;90:1073–1075. doi: 10.1080/080352501316978183. [DOI] [PubMed] [Google Scholar]
- 36.Mrukowicz JZ et al. Epidemiology and impact of rotavirus diarrhoea in Poland. Acta Paediatrica. 1999;426:53–60. doi: 10.1111/j.1651-2227.1999.tb14327.x. [DOI] [PubMed] [Google Scholar]
- 37.Gendrel D et al. Coincidental outbreaks of rotavirus and respiratory syncytial virus in Paris: a survey from 1993 to 1998 [in French] Archives de Pédiatrie. 1999;6:735–739. doi: 10.1016/s0929-693x(99)80355-8. [DOI] [PubMed] [Google Scholar]
- 38.Medici MC et al. Epidemiological aspects of human rotavirus infection in children hospitalized with acute gastroenteritis in an area of northern Italy. Acta Bio-Medica de l'Ateneo Parmense. 2004;75:100–106. [PubMed] [Google Scholar]
- 39.Berner R, Schumacher RF, Forster J. Survey on rotavirus infection in a German pediatric hospital. European Journal of Clinical Microbiology and Infectious Disease. 1997;16:479–481. doi: 10.1007/BF02471919. [DOI] [PubMed] [Google Scholar]
- 40.Román E et al. Acute viral gastroenteritis: proportion and clinical relevance of multiple infections in Spanish children. Journal of Medical Microbiology. 2003;52:435–440. doi: 10.1099/jmm.0.05079-0. [DOI] [PubMed] [Google Scholar]
- 41.Gil A et al. Burden of hospitalizations attributable to rotavirus infection in children in Spain, period 1999–2000. Vaccine. 2004;22:2221–2225. doi: 10.1016/j.vaccine.2003.11.037. [DOI] [PubMed] [Google Scholar]
- 42.Cilla G et al. Hospitalizations for rotavirus gastroenteritis in Gipuzkoa (Basque Country), Spain. Emerging Infectious Diseases. 1999;5:834–835. doi: 10.3201/eid0506.990619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Noel JS, Beards GM, Cubitt WD. Epidemiological survey of human rotavirus serotypes and electropherotypes in young children admitted to two children's hospitals in Northeast London from 1984 to 1990. Journal of Clinical Microbiology. 1991;20:2213–2219. doi: 10.1128/jcm.29.10.2213-2219.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Moulin F et al. Hospitalization for acute community-acquired rotavirus gastroenteritis: a 4-year study [in French] Archives de Pédiatrie. 2002;9:255–261. doi: 10.1016/s0929-693x(01)00761-8. [DOI] [PubMed] [Google Scholar]
- 45.Frühwirth M et al. Economic impact of community- and nosocomially acquired rotavirus gastroenteritis in Austria. Pediatric Infectious Disease Journal. 2001;20:184–188. doi: 10.1097/00006454-200102000-00013. [DOI] [PubMed] [Google Scholar]
- 46.Hammerschmidt T, Gartner B. Acute rotavirus gastroenteritis: burden of disease and cost of illness among young children in Germany. Value in Health. 2004;7:762. [Google Scholar]
- 47.Noel JS et al. Impact of rotavirus infection on a paediatric hospital in the East End of London. Journal of Clinical Pathology. 1994;47:67–70. doi: 10.1136/jcp.47.1.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Harrington M, Butler K, Cafferkey M. Rotavirus infection in hospitalised children: incidence and impact on healthcare resources. Irish Journal of Medical Science. 2003;172:33–36. doi: 10.1007/BF02914784. [DOI] [PubMed] [Google Scholar]
- 49.Hill C et al. Rotavirus gastroenteritis among paediatric patients at Tralee General Hospital. Irish Medical Journal. 2000;93:274–277. [PubMed] [Google Scholar]
- 50.Thuret A et al. Prospective follow-up of hospital-acquired diarrhoea in 28 paediatric wards of the south-east part of France during a winter season. Pathology and Biology. 2004;52:131–137. doi: 10.1016/j.patbio.2003.06.005. [DOI] [PubMed] [Google Scholar]
- 51.Piednoir E et al. Economic impact of healthcare-associated rotavirus infection in a paediatric hospital. J Hosp Infect. 2003;55:190–195. doi: 10.1016/j.jhin.2003.07.002. [DOI] [PubMed] [Google Scholar]
- 52.Roman Riechmann E et al. Nosocomial gastroenteritis and asymptomatic rotavirus and astrovirus infection in hospitalized children [in Spanish] Anales de Pediatria (Barcelona) 2004;60:337–343. doi: 10.1016/s1695-4033(04)78280-6. [DOI] [PubMed] [Google Scholar]
- 53.Gianino P et al. Incidence of nosocomial rotavirus infections, symptomatic and asymptomatic, in breast-fed and non-breast-fed infants. Journal of Hospital Infection. 2002;50:13–17. doi: 10.1053/jhin.2001.1129. [DOI] [PubMed] [Google Scholar]
- 54.Bennet R et al. Nosocomial gastroenteritis in two infant wards over 26 months. Acta Paediatrica. 1995;84:667–671. doi: 10.1111/j.1651-2227.1995.tb13724.x. [DOI] [PubMed] [Google Scholar]
- 55.Grassano Morin A et al. Nosocomial intestinal infections in an infant ward. The importance of phone inquiries of the families [in French] Archives de Pédiatrie. 2000;7:1059–1063. doi: 10.1016/s0929-693x(00)00313-4. [DOI] [PubMed] [Google Scholar]
- 56.Pina P et al. Nosocomial rotavirus infections in a general paediatric ward: epidemiology, molecular typing and risk factors [in French] Archives de Pédiatrie. 2000;7:1050–1058. doi: 10.1016/s0929-693x(00)00312-2. [DOI] [PubMed] [Google Scholar]
- 57.Rouget F et al. Evaluation of a prevention programme against nosocomial rotavirus infections in a paediatric ward [in French] Archives de Pédiatrie. 2000;7:948–954. doi: 10.1016/s0929-693x(00)90008-3. [DOI] [PubMed] [Google Scholar]
- 58.Sermet-Gaudelus I et al. Rotavirus nosocomial infection in pediatric units. A multicentric observation study. Pathologie Biologie. 2004;52:4–10. doi: 10.1016/j.patbio.2003.04.002. [DOI] [PubMed] [Google Scholar]
- 59.Jusot J-F et al. Reported measures of hygiene and incidence rates for hospital-acquired diarrhea in 31 French pediatric wards: is there any relationship. Infection Control and Hospital Epidemiology. 2003;24:520–525. doi: 10.1086/502238. [DOI] [PubMed] [Google Scholar]
- 60.Rytlewska M et al. Epidemiological and clinical characteristics of rotaviral diarrhoea in children from Gdansk, Gdynia and Sopot. Medical Science Monitor. 2000;6:117–122. [PubMed] [Google Scholar]
- 61.Crowley DS, Ryan MJ, Wall PG. Gastroenteritis in children under 5 years of age in England and Wales. Communicable Disease Report. CDR Review. 1997;7:R82–R86. [PubMed] [Google Scholar]
- 62.Bresee JS et al. First report from the Asian Rotavirus Surveillance Network. Emerging Infectious Diseases. 2004;10:988–995. doi: 10.3201/eid1006.030519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hsu VP et al. Use of active surveillance to validate international classification of diseases code estimates of rotavirus hospitalizations in children. Pediatrics. 2005;115:78–82. doi: 10.1542/peds.2004-0860. [DOI] [PubMed] [Google Scholar]
- 64.Fischer TK, Bresee JS, Glass RI. Rotavirus vaccines and the prevention of hospital-acquired diarrhea in children. Vaccine. 2004;22S:S49–S54. doi: 10.1016/j.vaccine.2004.08.017. [DOI] [PubMed] [Google Scholar]
- 65.Ruuska T, Vesikari T. Rotavirus disease in Finnish children: use of numerical scores for clinical severity of diarrheal episodes. Scandinavian Journal of Infectious Disease. 1990;22:259–267. doi: 10.3109/00365549009027046. [DOI] [PubMed] [Google Scholar]
- 66.Riordan FAI, Quigley T. Estimating hospital admissions due to rotavirus gastroenteritis from hospital episode statistics. Journal of Infection. 2004;49:13–16. doi: 10.1016/j.jinf.2004.02.006. [DOI] [PubMed] [Google Scholar]


