SUMMARY
The objective of the study was to determine the prevalence of smear-positive tuberculosis (TB) in a rural area in Bangladesh at Matlab. A TB surveillance system was established among 106 000 people in rural Bangladesh at Matlab. Trained field workers interviewed all persons aged ⩾15 years to detect suspected cases of TB (cough>21 days) and sputum specimens of suspected cases were examined for acid-fast bacilli (AFB). Of 59 395 persons interviewed, 4235 (7·1%) had a cough for >21 days. Sputum specimens were examined for AFB from 3834 persons, 52 (1·4%) of them were positive for AFB. The prevalence of chronic cough and sputum positivity were significantly higher among males compared to females (P<0·001). The population-based prevalence rate of smear-positive TB cases was 95/100 000 among persons aged ⩾15 years. Cases of TB clustered geographically (relative risk 5·53, 95% CI 3·19–9·59). The high burden of TB among rural population warrants appropriate measures to control TB in Bangladesh. The higher prevalence of persistent cough and AFB-positive sputum among males need further exploration. Factors responsible for higher prevalence of TB in clusters should be investigated.
INTRODUCTION
About 10 million new cases of tuberculosis (TB) occur globally each year, 70% of new cases are aged between 15 and 59 years and there are about 3 million deaths [1]. A recent analysis of the global burden of TB revealed that Bangladesh ranked as the fourth highest among 212 countries in 2001 [2] having 300 000 new cases and around 70 000 deaths annually. However, there is limited systematically collected epidemiological data from Bangladesh. While directly observed treatment, short-course (DOTS) is provided by the government, effective therapy is hampered by the fact that only 32% of cases are detected [3]. The earliest TB prevalence survey conducted by the National Tuberculosis Control Programme (NTP) during 1987–1988 showed that 0·87% of the population aged ⩾15 years old had sputum positive for acid-fast bacillus (AFB). It was more prevalent among men (1·08%) than women (0·60%) and more so in urban (1·61%) than rural areas (0·80%) [4]. Recently, the annual incidence of TB in Bangladesh was estimated at 220/100 000 [5]. The available data on the impact of TB control measures on the prevalence of disease in Bangladesh is limited [6]. Lack of such data makes policy decisions and successful monitoring of programmes difficult.
The case notification rates in most of the countries including Bangladesh are higher for males than females. Globally, the ratio of female to male TB cases notified is 1/1·5–2·1 [7]. Around 70% more cases of males, as defined by positive smears and notified to the WHO, are diagnosed every year. The reasons for these gender differences are not clear. They may be due to differences in prevalence of infection, rate of progression from infection to disease, under-reporting of female cases or differences in access to services [8, 9].
High population density, extreme poverty and malnutrition create a substantial risk for infection with Mycobacterium tuberculosis in Bangladesh. Considering the scarcity of data on TB disease burden and an impending threat of a human immunodeficiency virus (HIV) epidemic [10] in Bangladesh, we carried out a population-based surveillance to precisely estimate the prevalence of TB in a rural area in Bangladesh.
METHODS
Study site and population
The study was conducted in rural Bangladesh at Matlab, where the International Centre for Diarrhoeal Disease Research, Bangladesh (ICDDR,B) has been maintaining a field research project since 1963. Matlab is a low-lying riverine area which lies 45 km south-east of Dhaka, the capital of Bangladesh. The principal occupations in the Matlab area are farming and fishing. Since 1966 a Health and Demographic Surveillance System (HDSS), which consists of regular cross-sectional censuses and longitudinal registration of important events, has been maintained in the area [11]. A Maternal, Child Health & Family Planning Programme (MCH-FP) has been in operation for half of the population of the HDSS area (current population of HDSS is ∼220 000) since 1978 and intensive research has been conducted in this population [12]. In the other half of the population routine government health services are available including case detection and treatment of TB under NTP. The study was conducted in the MCH-FP intervention area.
Surveillance and data collection
Each community health research worker (CHRW) in the MCH-FP area covers a population of around 1900. CHRWs visit each household monthly and are responsible for the recording of important events, collecting health information about diarrhoea, acute respiratory infections and breastfeeding, immunization of children, referral of severely ill children and mothers, etc. During their monthly visits between October and December 2001, the CHRWs asked about all individuals in the household aged ⩾15 years old who had symptoms meeting a case definition for pulmonary TB (cough >3 weeks). After obtaining written consent a history of illnesses and sociodemographic data was collected from all suspected TB cases (cough >3 weeks) by study health workers through home visits. Data collected included demographic and socioeconomic information, previous treatment for TB, contact with TB patients, BCG vaccination status, etc.
Referral and treatment
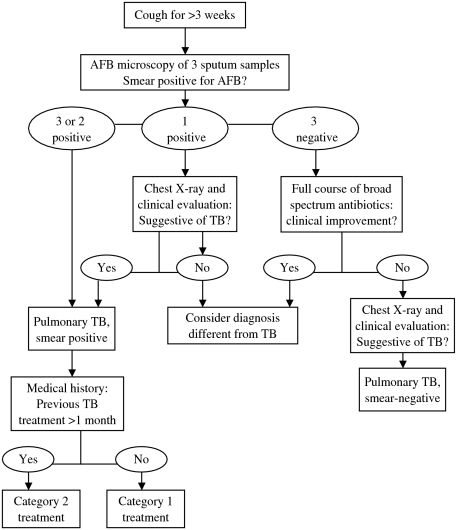
Field workers referred all suspected cases of TB to the Matlab Thana Health Complex (THC) for processing sputum for AFB. Some patients received a chest X-ray at the discretion of the physician. Three sputum specimens (two spot specimens and one morning specimen) were collected from each suspected TB patients on two consecutive days. All sputum specimens were examined for AFB at the Matlab THC and treatment was provided in each case. The existing National Tuberculosis Control Programme algorithm (Fig. 1) was used for the diagnosis and treatment of pulmonary TB [13]. All sputum smear-positive pulmonary TB cases were categorized depending on previous treatment, failure and relapse, and treated accordingly with first-line anti-TB drugs for a period of 8 months [13].
Fig. 1.
Algorithm for diagnosis of pulmonary tuberculosis. Category 1 treatment: 2 months (HRZE) and 6 months (HT). Category 2 treatment: 2 months (SHRZE), 1 month (HRZE) and 5 months (HRE). H, isoniazid; R, rifampicin; Z, pyrazinamide; E, ethambutol; T, thiacetazone; S, streptomycin. (Source: adapted from ref. [13].)
Geographical mapping process
The geographical clusters of higher prevalence of TB were defined from spatially smoothed data on the prevalence rate of TB. The spatially smoothed data were obtained for each of the bari (cluster of houses using a common courtyard) points by computing the prevalence rate within 1-km radius area of the point. We tried several sizes of radius, and observed that the 1-km area was the most suitable size to make spatially smoothed rates of the disease under the specific spatial arrangement of the bari points and the number of cases. There were 3773 baris in the study area, thus, the spatially smoothed rate was obtained for the 3773 sample points of the study area.
The spatially smoothed data of the sample points were used to interpolate the data at a regularly spaced interval for creating a surface map of the disease. A widely used geostatistical method called kriging was used to interpolate the data. Kriging is an optimal interpolator – its estimates are unbiased and have minimum variance [14, 15]. The method is based on the assumption of spatial autocorrelation of data, and the autocorrelation structure (spatial distribution of the data) is addressed through variogram modelling. The underlying assumption of the variogram is that two observations close together are more similar than those further apart. Using the variogram in combination with observed data, we used ordinary kriging to interpolate the data at regular intervals. An ordinary kriged estimate of a variable Z at a point s is the weighted average of
 |
Z(si) is the observed data value at points i, δi is the weight associated with the data at point i, which is obtained from a variogram modelling. Finally, a contour mapping technique [16] was used to take out the elevated surface (the surface cut-off of it was 1·1/1000) of higher prevalence of the disease, and defined the elevated surface as the cluster of higher prevalence of the disease. The methods were found suitable for defining higher prevalence areas for infectious diseases [17, 18].
Laboratory methods
A loopful of sputum was used to perform Ziehl–Neelsen staining following standard procedure [19]. Stained smear was examined under microscope in oil immersion and reported as described earlier [20].
Definitions
A case of pulmonary TB was defined as having at least two sputum specimens positive for AFB or one sputum positive for AFB and radiological abnormalities consistent with TB [21].
Data analysis
Data were entered using the software package FoxPro (Microsoft Corp., USA) and analysed by the stata statistical software (Release 8.0, Stata Corporation, College Station, TX, USA). The prevalence of smear-positive TB was calculated among those who had a cough and brought sputum for examination. The population-based rate was obtained by extrapolating the rates in 100 000 population aged ⩾15 years. The χ2 test was used to compare categorical values between the groups.
Quality control
All positive AFB and 10% of negative specimens at Matlab were rechecked in Dhaka by an experienced microbiologist (Z.R.). The overall agreement between the two tests was 96%. The ethical review committee of ICDDR,B approved the study.
RESULTS
Interviews were done with 59 395 (88·7% of 66 946) persons aged ⩾15 years old; the prevalence of cough >21 days was 7·1% (Table 1), and this was more common among males (9·2% vs. 5·5%, P<0·0001). When considering age, the highest proportion was observed among persons aged ⩾45 years (11·5%) and lowest among the 15–24 years age group (3·5%).
Table 1.
Age and gender distribution of patients with cough for >21 days, Matlab, 2001
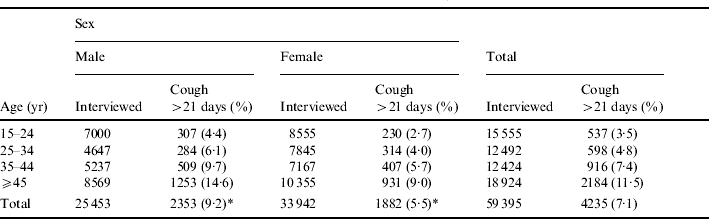
χ2 test, P<0·0001.
Sputum specimens were examined for AFB from 3834 persons with cough >21 days. About 48% of them had no schooling and 23% had secondary education (⩾6 years schooling), 12% had a history of taking BCG vaccine and 21% had reported ever history of TB in the family. The mean household income was about US$70 per month, the mean household size was 5·6 persons and 98% of the houses were made of corrugated iron roof and 70% with corrugated iron wall (data not shown). Among 3834 persons tested for AFB, 52 (1·4%) were positive (Table 2). Around 83% of AFB-positive cases were either not treated or treated for <1 month with anti-TB drugs before sputum samples were examined (data not shown), 5·8% were treated for <1 month. The sputum positivity was significantly higher among males compared to females (2·1% vs. 0·6%, P<0·001) (Table 2). The overall population-based prevalence of smear-positive TB was 95/100 000 population aged ⩾15 years; significantly higher in males than females (190 vs. 31/100 000, P<0·0001).
Table 2.
Age and gender distribution of AFB-positive cases, Matlab, 2001
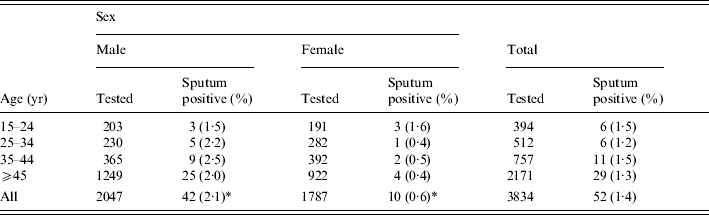
AFB, Acid-fast bacillus.
P<0·001.
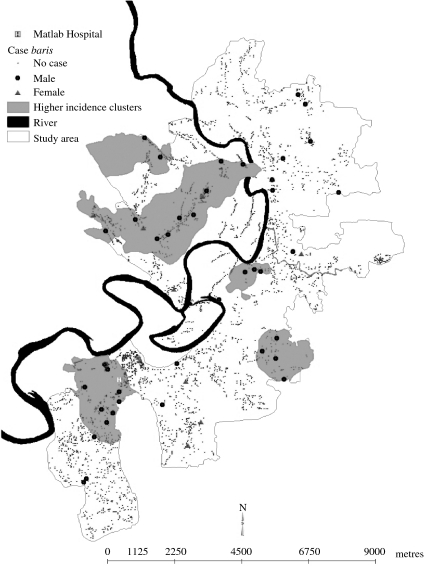
Figure 2 shows that the prevalent cases of TB were more likely to occur in some specific areas shown in the map (called clusters of higher prevalence of the disease) compared with the rest of the area [216 vs. 39/100 000; relative risk 5·53, 95% confidence interval (CI) 3·19–9·59, P<0·001].
Fig. 2.
Geographic clusters of increased risk of TB, Matlab study area, 2001.
DISCUSSION
The study confirms that TB is a substantial problem among persons aged ⩾15 years in rural Bangladesh at Matlab. The population-based rate of sputum smear-positive was 95/100 000 persons aged ⩾15 years old. The rate was much lower than the rate observed in the 1987–1988 national TB prevalence survey [4] in Bangladesh (870/100 000) but higher than a recently conducted study (44/100 000) [22]. Recent estimates (2004) from NTP reveals that the annual case detection rate is 41/100 000 population at Matlab where our study area is located (V. Begum, personal communication). Furthermore, DOTS was adopted in Bangladesh in 1993 and expanded countrywide within 5 years [23], but in urban areas only in the last 2–3 years. The limitation of the 1987–1988 survey [4] was that some of the survey areas were inaccessible and female respondents did not cooperate and provide sputum samples. Our study interviewed all persons to obtain coughing symptoms and repeat visits were made for absentees. However, Salim et al. [22] interviewed family members when individuals were absent at the time of visit. When we compared our rates with some Asian countries, we found that Korea had similar rate (93/100 000) [24] but the rates were higher in India (400/100 000) [25], Philippines (310/100 000) [26] and China (122/100 000) [27]. However, comparisons of prevalence rates between countries should be done with caution. The differences may be due to difference in survey methods, population and age groups studied and time periods. For example, multistage cluster sampling technique was used in some studies [4, 22, 24, 26] while others used random sampling [27] or included all samples [25]. The age groups studied were also different between studies and covered all ages [27], ⩾5 years [24, 25], ⩾10 years [26], ⩾12 years [22] or ⩾15 years [4]. There is no precise information on case detection rate from the urban areas in Bangladesh. The detection of TB cases in Bangladesh under NTP is done on the basis of number of cases reporting to DOTS centres.
The 7·1% prevalence of cough of >3 weeks duration (suspected TB) is somewhat consistent with the findings from other studies [4, 28–30]. The rate was 7·7% among the population aged ⩾10 years in the 1964–1966 survey [28] and 5·9% in the 1987–1988 survey in Bangladesh [4]. Hafez et al. [29] reported cough for ⩾4 weeks in 5·3% of individuals in a survey among a rural population in two villages. However, the rates were lower in a recent study in Bangladesh (2·6%) [22] and in a population-based study [30] in Vietnam (1·5%).
The population-based rate of smear-positive TB was almost six times higher in males than females in our study. Another study in Bangladesh revealed about three times higher sputum-positive cases in males compared to females (35·4 vs. 12·3/100 000) [22]. The male predominance for persistent cough and for AFB-positive sputum is consistent with data from other countries and could reflect occupational, behavioural or immunological contributions to risk [31–33]. One study in Bangladesh suggested that women have less access to public health clinics, and they are less likely to undergo sputum smear examination when they present with chronic cough [34]. The authors of the Bangladeshi study hypothesized that women might give poorer quality specimens than men which would be less likely to reveal AFB [34]. Studies from Vietnam and Zambia have also suggested that gender differences in diagnosis reflect inequities in health care [35, 36]. However, active case finding via household visits should have dramatically reduced the impact of inequity of services, raising the possibility that the gender differences we observed were not artifactual.
The finding of clustering of cases, while not unexpected, provides support for the concept of heightened surveillance for TB in areas where a case has been identified. In future studies attempts should be made to identify the factors associated with clustering of TB cases which should help to improve case detection. Cost-effective strategies are needed to improve case-detection rates so that a substantially higher proportion of cases are treated, minimizing the impact and spread of TB.
Considering that TB poses a high economic burden in the family and with the impending threat of a HIV epidemic, the NTP in Bangladesh considers it a high priority to increase the case detection rate from its current level of 32% [3].
We limited our study to persons aged ⩾15 years old since this is the target age group in the national TB control programme and sputum specimens are difficult to obtain in children. Thus, this study is unable to examine and highlight the impact of TB in the paediatric population in Bangladesh. However, we are conducting a study to develop a simple algorithm to diagnose TB among hospitalized children. If the scoring system is found to be predictive, it will have potential for diagnosis and treatment of TB among children in a hospital or clinic setting. TB cases receiving treatment but not having a cough for ⩾3 weeks at the time of the survey were not included in testing of sputum samples. Therefore, some prevalent cases might have been missed. Furthermore, sputum samples could not be examined in 13% of males and 5% of females since they could not attend Matlab THC. Repeated attempts were made by the field workers to bring them to Matlab for sputum examination. Detailed data from 34% of these persons were available. This shows that the characteristics of these persons were not different from those who provided sputum samples in terms of education levels, monthly household income, BCG vaccination status, mean household size, etc. The age distribution was similar in the 25–34 and 35–44 years age groups but different in the 15–24 and ⩾45 years age groups. We believe that this will not affect overall TB prevalence rates in our study since the rate is similar in different age groups (Table 2). The prevalent cases in this study were sputum positive at the time of the survey irrespective of treatment status. Furthermore, the study was not designed to assess the impact of the NTP on the transmission of TB.
The high burden of TB among the rural population warrants appropriate measures to control TB in Bangladesh. The higher prevalence of persistent cough and AFB-positive sputum among males need further exploration. Factors responsible for higher prevalence of TB in clusters should be investigated. Appropriate strategies for prevention (e.g education and behaviour modification), targeted diagnosis and treatment are needed to strengthen TB control activities in Bangladesh.
ACKNOWLEDGEMENTS
The study was conducted at the ICDDR,B, Centre for Health and Population Research with the financial support of USAID (US Agency for International Development, cooperative agreement no. HRN-A-00-96-90005-00) and the Department for International Development (DFID). ICDDR,B acknowledge with gratitude the commitment of USAID and DFID to the Centre's research efforts. We acknowledge the contribution of the National Tuberculosis Control Programme (NTP) of Bangladesh for their support in conducting this study.
DECLARATION OF INTEREST
None.
REFERENCES
- 1.Dolin PJ, Raviglione MC, Kochi A. Global tuberculosis incidence and mortality during 1990–2000. Bulletin of the World Health Organization. 1994;72:213–220. [PMC free article] [PubMed] [Google Scholar]
- 2.World Health Organization Geneva: 2003. . Global tuberculosis control: surveillance, planning, financing. WHO Report 2003 (WHO/CDS/TB/2003.316). : WHO, [Google Scholar]
- 3.World Health Organization New Delhi, India: 2003. . Tuberculosis control in the south-east Asia region: the regional report 2003 (SEA/TB/260). : WHO, [Google Scholar]
- 4.Director General Health Services (DGHS) Dhaka: 1989. . Report on the national prevalence survey on tuberculosis in Bangladesh, 1987–88. : Ministry of Health and Family Welfare, Government of Bangladesh, [Google Scholar]
- 5.Kumaresan JA, Raviglione MC, Murray CJL, Murray CLJ, Lopez AD. Global Health Statistics: global burden of disease and injury series. Vol. 2. Boston, MA: Harvard University Press; 1996. Tuberculosis; pp. 142–147. , vol. : pp. [Google Scholar]
- 6.Islam MN 1995. . Impact of TB control project on prevalence of the disease in rural Bangladesh. Bangladesh Rural Advancement Committee Report,
- 7.Diwan VK, Thorson A. Sex, gender, and tuberculosis. Lancet. 1999;353:1000–1001. doi: 10.1016/S0140-6736(99)01318-5. [DOI] [PubMed] [Google Scholar]
- 8.Holmes CB, Hausler H, Nunn P. A review of sex differences in the epidemiology of tuberculosis. International Journal of Tuberculosis and Lung Disease. 1998;2:96–104. [PubMed] [Google Scholar]
- 9.Borgdorff MW Geneva: 1999. . Gender and tuberculosis in Bangladesh: evidence for gender bias in tuberculosis case detection and proposals for further study. Mission report (WHO/CDS/CPC/TB/99.266). : WHO, [Google Scholar]
- 10.International Centre for Diarrhoeal Disease Research, Bangladesh (ICDDR,B) HIV surveillance in Bangladesh. Health and Science Bulletin. 2003;1:1–7. [Google Scholar]
- 11.International Centre for Diarrhoeal Disease Research, Bangladesh (ICDDR,B) 1978. p. 9. . Demographic Surveillance System – Matlab, methods and procedures. ICDDR,B Scientific Report, : p.
- 12.Bhatia S et al. The Matlab Family Planning Health Services Project. Studies in Family Planning. 1980;11:202–212. [PubMed] [Google Scholar]
- 13.Government of Bangladesh Directorate General of Health Services, National Tuberculosis Control Programme; Dhaka, Bangladesh: 1999. pp. 1–11. . Tuberculosis Control Programme in Bangladesh. Technical outline. : pp. [Google Scholar]
- 14.Cressie NAC. Statistics for Spatial Data. New York: John Wiley & Sons Inc.; 1991. pp. 119–143. : pp. [Google Scholar]
- 15.Oliver MA, Webster R. Kriging: a method of interpolation for geographical information systems. International Journal of Geographical Information Systems. 1990;4:313–332. [Google Scholar]
- 16.Surfer Golden Software, Inc.; Colorado, USA: 1999. . Contouring and 3D surface mapping for scientist and engineers. [Google Scholar]
- 17.Ali M et al. Are the environmental niches of Vibrio cholerae O139 different from that of Vibrio cholerae El Tor. International Journal of Infectious Diseases. 2001;5:214–219. doi: 10.1016/s1201-9712(01)90074-8. [DOI] [PubMed] [Google Scholar]
- 18.Ali M et al. The spatial epidemiology of cholera in an endemic area of Bangladesh. Social Science Medicine. 2002;55:1015–1024. doi: 10.1016/s0277-9536(01)00230-1. [DOI] [PubMed] [Google Scholar]
- 19.Della-Latta P, Weitzman I, Isenberg HD. Essential Procedures for Clinical Microbiology. Washington, DC: American Society of Microbiology; 1998. Mycobacteriology; pp. 169–203. : pp. [Google Scholar]
- 20.World Health Organization Geneva: WHO; 1998. . Laboratory services in tuberculosis control: microscopy part II (WHO/TB/98.258). [Google Scholar]
- 21.World Health Organization Geneva: WHO; 2003. . Treatment of tuberculosis: guidelines for national programmes (WHO/CDS/TB/2003.313). [Google Scholar]
- 22.Salim MAH et al. Gender differences in tuberculosis: a prevalence survey done in Bangladesh. International Journal of Tuberculosis and Lung Disease. 2004;8:952–957. [PubMed] [Google Scholar]
- 23.Director General Health Services (DGHS) Dhaka: Ministry of Health and Family Welfare, Government of Bangladesh; 2004. . National guidelines and operational manual for tuberculosis control. [Google Scholar]
- 24.Hong YP et al. The seventh nationwide tuberculosis prevalence survey in Korea, 1995. International Journal of Tuberculosis and Lung Disease. 1998;2:27–36. [PubMed] [Google Scholar]
- 25.Chadha VK. Epidemiological situation of tuberculosis in India. Journal of the Indian Medical Association. 2003;101:144–147. [PubMed] [Google Scholar]
- 26.Tupasi TE et al. The 1997 nationwide tuberculosis prevalence survey in the Philippines. International Journal of Tuberculosis and Lung Disease. 1999;3:471–477. [PubMed] [Google Scholar]
- 27.China Tuberculosis Control Collaboration. The effect of tuberculosis in China. Lancet. 2004;364:417–422. doi: 10.1016/S0140-6736(04)16764-0. [DOI] [PubMed] [Google Scholar]
- 28.Government of Bangladesh 1973. . The report of tuberculosis survey of Bangladesh. National Tuberculosis control and research project. Ministry of Health and Family Planning, Government of Bangladesh,
- 29.Hafez MA et al. A study on the prevalence of tuberculosis in a rural community in Bangladesh. Bangladesh Medical Research Council Bulletin. 1991;XVII:23–29. [PubMed] [Google Scholar]
- 30.Thorson A, Hoa NP, Long NH. Health seeking behaviour of individuals with a cough of more than three weeks. Lancet. 2000;356:1823–1824. doi: 10.1016/s0140-6736(00)03241-4. [DOI] [PubMed] [Google Scholar]
- 31.Hudelson P. Gender differentials in tuberculosis: the role of socio-economic and cultural factors. Tubercle Lung Disease. 1996;77:391–400. doi: 10.1016/s0962-8479(96)90110-0. [DOI] [PubMed] [Google Scholar]
- 32.Borgdorff MW et al. Gender and tuberculosis: a comparison of prevalence surveys with notification data to explore sex differences in case detection. International Journal of Tuberculosis and Lung Disease. 2000;4:123–132. [PubMed] [Google Scholar]
- 33.Yamasaki-Nakagawa M et al. Gender difference in delays to diagnosis and health care seeking behaviour in a rural area of Nepal. International Journal of Tuberculosis and Lung Disease. 2001;5:24–31. [PubMed] [Google Scholar]
- 34.Begum V et al. Tuberculosis and patient gender in Bangladesh: sex differences in diagnosis and treatment outcome. International Journal of Tuberculosis and Lung Disease. 2001;5:604–610. [PubMed] [Google Scholar]
- 35.Thorsan A, Diwan VK. Gender inequalities in tuberculosis: aspects of infection, notification rates, and compliance. Current Opinion in Pulmonary Medicine. 2001;7:165–169. doi: 10.1097/00063198-200105000-00009. [DOI] [PubMed] [Google Scholar]
- 36.Needhan DM et al. Socio-economic, gender and health services factors affecting diagnostic delay for tuberculosis patients in urban Zambia. Tropical Medicine and International Health. 2001;6:256–259. doi: 10.1046/j.1365-3156.2001.00709.x. [DOI] [PubMed] [Google Scholar]