SUMMARY
Female sex workers in Europe have low levels of sexually transmitted infections, attributable to condom use. The aim of this paper is to describe the seroepidemiology of HSV-1 and HSV-2 in female sex workers in London by using a 15-year prospective study of 453 sex workers. The seroprevalence of HSV-1 was 74·4% and independently associated with birth in a ‘transitional country’ (OR 5·4, 95% CI 1·61–18·20). The seroprevalence of HSV-2 was 60% and declined over time; it was also independently associated with time in sex work (OR 2·12, 95% CI 1·23–3·65) and birth in a ‘developing country’ (OR 2·95, 95% CI 1·34–6·48). We show that a cohort of sex workers with extensive condom use and little known sexually transmitted infection have high levels of HSV-1 and HSV-2 infection, suggesting that condoms may not be universally protective. Sex workers are candidates for HSV vaccine efficacy or intervention studies.
INTRODUCTION
The incidence of diagnosed genital herpes in the United Kingdom has steadily increased over recent decades [1, 2]. Genital herpes has a wide geographic and behavioural distribution that extends well beyond populations considered to be at high risk for other infections such as gonorrhoea. Recently a significant variation in the seroprevalence of HSV in the general population across Europe has been shown [3]. General populations, cohorts of young females and patients attending hospital have been investigated but the seroprevalence of HSV-1 and HSV-2 in sex workers is not known. In the United Kingdom, for example, the seroprevalence of HSV-2 in the general population is 8% [4] and in genitourinary clinic attendees it is 23% [4]. Seroprevalence rates in sex workers in non-European countries range from 61% [5] to 90% in Zaire [6]. Historically sex workers are a highly mobile and distinct group in terms of sexual behaviour compared to the general population.
Any successful interventions to control genital herpes, whether through vaccination, therapy or behaviour change, would have to reach a large proportion of the sexually active population. There are a number of likely limitations: obstacles to the widespread delivery and acceptance of a vaccine against a sexually transmitted infection (STI) [7], the identification of appropriate individuals for suppressive therapy to reduce onward transmission, and the relative ineffectiveness of condoms against HSV transmission [8].
Suppressive therapy has mainly been indicated for prevention of recurrent attacks, but has also been shown to have some impact on transmission within HSV discordant partnerships [9]. It is unlikely that this would have any impact at a population level unless targeted at groups that contribute disproportionately to transmission. These include people with serological evidence of infection who remain undiagnosed [10, 11].
We, therefore, investigated the epidemiology of HSV infection in a cohort of female sex workers in London and discuss whether they are an appropriate group for screening and intervention. This particular cohort has been well described, and has high rates of partner change, high rates of condom use and relatively low rates of bacterial STI and HIV infection [12, 13]. The cohort has a prevalence of 17% and an incidence of 6·5/100 person-years for clinical genital herpes.
METHODS
The Praed Street Project is a clinical and health promotion service for female sex workers in West London. From 1985 to 2000, new attendees were invited to participate in research into health and risk behaviour. Detailed methods are reported elsewhere [12, 13]; briefly, baseline information is collected through interview covering social, sexual and medical history. Data on country of birth were broadly categorized as United Kingdom, developed, developing, and transitional (economies of the former Soviet states and east/central Europe). Time in sex work was calculated from recruitment data and reported date of first sex work. Information on condom use was ascertained through interview with questions on the previous month for male non-paying partners (boyfriends) and previous week (or most recent week worked) for clients. For the purposes of this analysis condom use was collapsed into two categories: always (100%) and not always (any<100%).
After giving informed consent, women being tested for HIV antibodies donated additional serum for storage. Participants were invited to re-attend the clinic for regular screening or when prompted by symptoms or other concerns. Sequentially stored serum samples were screened for IgG antibody to HSV-1 and HSV-2 using type-specific ELISAs routinely used by the UK Health Protection Agency [14].
Data were stored in a database, ensuring anonymity, and analysed using SPSS version 12 software (SPSS Inc., Chicago, IL, USA). Univariate analysis was carried out using the χ2 test for categorical variables. Incidence was calculated for those who were initially seronegative and who had more than one sample over time, using the denominator of total time from first test, censored at the time of first positive or last negative test. Logistic regression modelling was used to identify predictors for prevalent HSV-1 and HSV-2 infections; variables were retained and removed at the 5% level. The likelihood ratio test and Wald statistic were used to assess whether to remove or retain variables in the model.
Sampling bias was explored by comparing behaviour data (including clinical HSV history) of those who had serological tests with those who did not provide samples for storage. The study was approved by the local research ethics committee.
RESULTS
Baseline interview data and serological results were available for 453 (37%) of 1217 women seen between 1985 and 2000. The majority of the remaining 764 women (512, 67%) did not have a baseline HIV test and, for the remainder, we had no consent for storage of sera (many were not asked due to pressure of time in the clinic), there were insufficient stored sera, or the serological results were equivocal. We explored the potential of selection bias resulting from the inclusion of less than half of project attendees but found no significant differences in demographic, medical or risk characteristics between those who had samples and those who did not (see Table 1).
Table 1.
Exploration of potential selection bias: characteristics of sex workers who had serum stored compared with those who did not

The seroprevalence of HSV-1 was 337/451 [74·4%, 95% confidence interval (CI) 71·1–78·9] and of HSV-2, 272/453 (60·0%, 95% CI 55·5–64·5). In total, 441 women had results available for both as shown in Table 2. Those who were HSV-1 positive had a slightly lower prevalence of HSV-2 but this was not statistically significant.
Table 2.
Association between HSV-1 and HSV-2 seropositivity
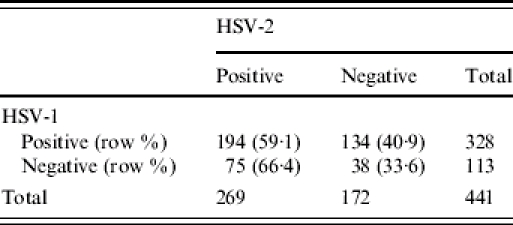
OR 0·734 (95% CI 0·47–1·15).
Table 3 shows factors associated with HSV-1 and HSV-2 antibodies. HSV-1 increased with age, was higher in women from developing countries or from countries in transition, and increased slightly over time. In multivariate analysis with a model testing these variables, being born in a country in transition was associated with a fivefold increase in risk [odds ratio (OR) 5·4, 95% CI 1·61–18·20, P=0·006], while increasing age remained of borderline significance (OR 1·04 for each year older, 95% CI 1·00–1·08, P=0·05), and year of recruitment was not retained.
Table 3.
Factors associated with antibodies to HSV-1 and HSV-2, univariate analysis
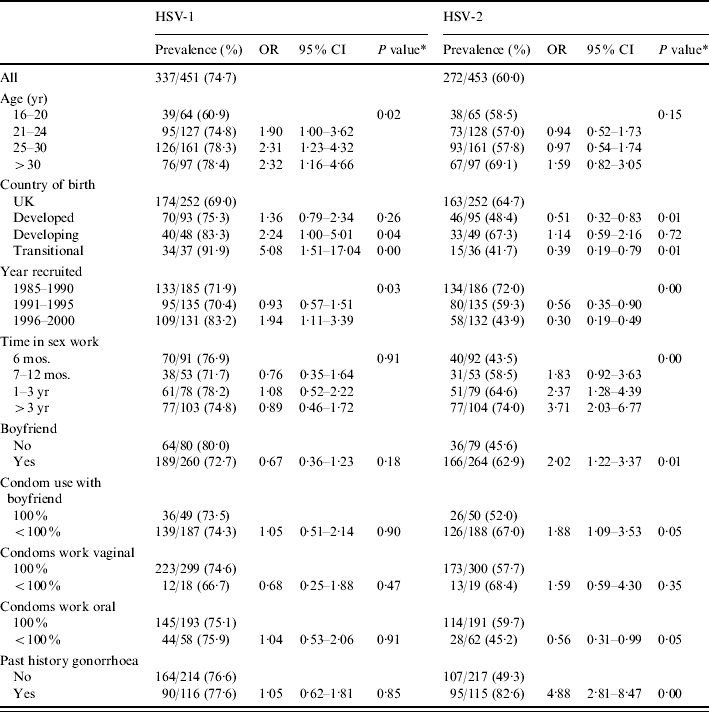
P value for trend presented for year recruited, age group and time in sex work.
In contrast, HSV-2 infection declined steeply with time, from 72% of women recruited in the 1980s to 44% of those recruited at the end of the 1990s. The prevalence was highest in women who were born in a developing country and lowest in those from developed countries other than the United Kingdom. There was an increase with time in sex work from 55% of those working <6 months to 74% of those working for >3 years. Rates were also increased in women with a history of gonorrhoea, with a boyfriend at first visit, and slightly lower in those who did not use condoms consistently for oral sex with clients. To adjust for confounding particularly between year of recruitment, country of origin and time in sex work, we developed a logistic regression model. Being recruited later was independently protective with a 13% decrease for each year of entry (OR 0·87, 95% CI 0·82–0·93, P<0·001), being born in a developing county increased the risk threefold (OR 2·95, 95% CI 1·34–6·48, P=0·007), and working >6 months doubled the risk (OR 2·12, 95% CI 1·23–3·65, P=0·007).
Evidence of seroconversion was seen in three of 28 women who were initially HSV-2 negative and had later samples. Those women contributed 102·6 years of follow-up; an incidence of 2·9 (95% CI 0·61–6·9) per 100 person-years. There were too few data to perform further analysis on this group.
Of the 272 women with antibodies to HSV-2 at baseline, only 32 (11·8%) reported a history of genital herpes infection, suggesting that they were undiagnosed, asymptomatic or had latent infection. A further eight women with a history of genital herpes had antibodies to HSV-1 only.
DISCUSSION
This is the first time that the seroprevalence of HSV-1 and HSV-2 has been described in female sex workers in Europe. Compared with previous studies of unselected patients attending sexually transmitted disease clinics in the United Kingdom, these sex workers have rates of HSV-2 three times higher and a 1·2-fold increase in HSV-1 [15].
The relatively high prevalence of HSV-1 and the small increase over time is likely to reflect the demographics of the population. The strongest association of seropositivity for HSV-1 was with origin in countries of the former Soviet Union and Eastern Europe where the background prevalence is higher [3]. In contrast, there was a steep decline in the prevalence of HSV-2 over time, and infection was independently associated with being born in a developing country and working for >6 months in the sex industry. The association with duration of sex work contrasts with earlier reports from this population where STI risks were associated primarily with non-commercial sexual contacts [12, 13].
Using this cross-sectional study design, we were unable to explore the relationship between condom use and HSV-2; our condom use data relate to the very recent past while HSV-2 could have been acquired at any time. However, long-term studies in this population show high levels of sustained condom use at work and so it is perhaps surprising that levels of HSV-2 are so high; particularly as condoms may at least be partially protective against HSV [16]. Our data suggest that HSV-2 antibodies remain a broad marker for cumulative sexual activity, even where condom use is widespread [4].
The changing prevalence of these herpes viruses over time requires further investigation to be fully explained. The changing demography of sex workers [12] in London may explain the small rise in HSV-1, but does not fully explain the decline in HSV-2. More recently, women attending the project reported shorter time in sex work, yet the temporal decline persists after controlling for this factor. The decline in HSV-2 coincides with higher rates of HSV-1 in a way that is consistent with findings from STI clinics linking HSV-1 transmission with oro-genital sex [4, 17]. It was not possible, however, to demonstrate this connection in our study as we did not have data on the site of clinical herpes. It has been suggested that HSV-1 is partially protective against HSV-2 [18] and given that HSV-1 is largely transmitted in childhood [19] the shift to a higher HSV-1 prevalent population could result in less HSV-2 transmission.
There are a number of limitations to this study, including the small number of participants each year and the potential sampling bias, with only a proportion of sex workers having serum stored and tested. We did not identify any systematic bias, but this does not exclude the possibility. The other major limitation relates to the design of the study, with most data coming from the baseline. We had too few longitudinal data to analyse risk factors for incidence, in part due to the high prevalence of infection at baseline.
The implications of this study are first that sex workers are at increased risk of acquiring herpes simplex infection despite sustained high levels of condom use. Even systematic use of condoms should be considered safer sex not safe sex. Sex workers need to be advised that condoms are not fully protective. Second, the overwhelming majority (88%) of HSV-2- positive women in this group did not have clinically apparent genital herpes; if these individuals are shedding the virus then their sexual partners are at risk of acquisition, including through oro-genital and protected vaginal or anal sex.
Finally, should we be advocating screening for HSV-2 infection in this population? Screening for HSV remains contentious, and would be particularly difficult for sex workers who are already stigmatized as ‘diseased’, and work hard to avoid infections and dispel these labels [20]. Evidence of infection of unknown origin, duration or implications for transmission would be difficult to manage without adding to stigma. Screening followed by counselling and suppressive therapy (for the duration of time in sex work) would be time consuming and costly, and the benefits would be difficult to establish [8]. In contrast, a vaccine that prevents acquisition may be of great benefit both to sex workers and to their sexual partners if given early in a sex worker's career; sex workers should be considered for efficacy or intervention studies for candidate HSV vaccines.
ACKNOWLEDGEMENTS
Thanks to all participants, and to the many collaborators who have contributed to this work over the years including John Clarke of the Jefferiss Laboratories, Anna Pallecaros and other staff of the Praed Street Project and the Jefferiss Wing Clinic, and to Reija Koukakis for assistance with data handling and preliminary analysis. Funding for this study was provided by the Wellcome Trust (grant no. 053592); earlier data were collected in studies funded by the Medical Research Council, AVERT, North West Thames Regional Health Authority and the Jefferiss Research Trust.
DECLARATION OF INTEREST
None.
REFERENCES
- 1.Health Protection Agency Annual Report www.hpa.org.uk/infections/topics_az/hiv_and_sti/publications/sti_report2002_tables.pdf. www.hpa.org.uk/infections/topics_az/hiv_and_sti/publications/sti_report2002_tables.pdf . Focus on Prevention of HIV and other Sexually Transmitted Infections in the United Kingdom in 2003. November 2004 ( ). Accessed 5 July 2005.
- 2.Brown AE et al. Recent trends in HIV and other STIs in the United Kingdom: data to the end of 2002. Sexually Transmitted Infections. 2004;80:159–166. doi: 10.1136/sti.2004.009571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pebody RG et al. The seroepidemiology of herpes simplex virus type 1 and 2 in Europe. Sexually Transmitted Infections. 2004;80:185–191. doi: 10.1136/sti.2003.005850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cowan FM et al. Antibody to herpes simplex virus type 2 as serological marker of sexual lifestyle in populations. British Medical Journal. 1994;309:1325–1329. doi: 10.1136/bmj.309.6965.1325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Conde-Glez CJ et al. Analysis of herpes simplex virus 1 and 2 infection in women with high risk sexual behaviour in Mexico. International Journal of Epidemiology. 1999;28:571–576. doi: 10.1093/ije/28.3.571. [DOI] [PubMed] [Google Scholar]
- 6.Nzila N et al. HIV and other sexually transmitted diseases among female prostitutes in Kinshasa. AIDS. 1991;5:715–721. doi: 10.1097/00002030-199106000-00011. [DOI] [PubMed] [Google Scholar]
- 7.Davis K et al. Human papillomavirus vaccine acceptability among parents of 10- to 15-year-old adolescents. Journal of Lower Genital Tract Disease. 2004;8:188–194. doi: 10.1097/00128360-200407000-00005. [DOI] [PubMed] [Google Scholar]
- 8.Wald A et al. Effect of condoms on reducing the transmission of herpes simplex virus type 2 from men to women. Journal of the American Medical Association. 2002;285:3100–3106. doi: 10.1001/jama.285.24.3100. [DOI] [PubMed] [Google Scholar]
- 9.Corey L et al. Once-daily valacyclovir to reduce the risk of transmission of genital herpes. New England Journal of Medicine. 2004;350:11–20. doi: 10.1056/NEJMoa035144. [DOI] [PubMed] [Google Scholar]
- 10.Wald A et al. Reactivation of genital herpes simplex virus type 2 infection in asymptomatic seropositive persons. New England Journal of Medicine. 2000;342:844–850. doi: 10.1056/NEJM200003233421203. [DOI] [PubMed] [Google Scholar]
- 11.Tetrault I, Boivin G. Recent advances in management of genital herpes. Canadian Family Physician. 2000;46:1622–1629. [PMC free article] [PubMed] [Google Scholar]
- 12.Ward H et al. Declining prevalence of STI in the London sex industry, 1985 to 2002. Sexually Transmitted Infections. 2004;80:374–378. doi: 10.1136/sti.2003.009068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ward H, Day S, Weber J. Risky business: health and safety in the sex industry over a 9-year period. Sexually Transmitted Infections. 1999;75:340–343. doi: 10.1136/sti.75.5.340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vyse A et al. The burden of infection with HSV-1 and HSV-2 in England and Wales: implications for the changing epidemiology of genital herpes. Sexually Transmitted Infections. 2000;76:183–187. doi: 10.1136/sti.76.3.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Narouz N et al. Genital herpes serotesting: a study of the epidemiology and patients' knowledge and attitude among STD clinic attenders in Coventry, UK. Sexually Transmitted Infections. 2003;79:35–41. doi: 10.1136/sti.79.1.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wald A et al. The relationship between condom use and herpes simplex virus acquisition. Annals of Internal Medicine. 2005;143:707–713. doi: 10.7326/0003-4819-143-10-200511150-00007. [DOI] [PubMed] [Google Scholar]
- 17.Brugha R et al. Genital herpes infection: a review. International Journal of Epidemiology. 1997;26:698–709. doi: 10.1093/ije/26.4.698. [DOI] [PubMed] [Google Scholar]
- 18.Looker KJ, Garnett GP. A systematic review of the epidemiology and interaction of herpes simplex virus types 1 and 2. Sexually Transmitted Infections. 2005;81:103–107. doi: 10.1136/sti.2004.012039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Scoular A et al. Longitudinal study of genital infection by herpes simplex virus type 1 in western Scotland over 15 years. British Medical Journal. 2002;324:1366–1367. doi: 10.1136/bmj.324.7350.1366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Day S, Ward H., Day S, Ward H. Sex Work, Mobility and Health in Europe. London: Kegan Paul; 2004. Approaching health through the prism of stigma: a longer term perspective; pp. 161–178. : pp. [Google Scholar]


