SUMMARY
A total of 113 strains of Shigella dysenteriae type 2 isolated from patients attending the Dhaka diarrhoea treatment centre of ICDDR,B: Centre for Health and Population Research during the period 1999–2004 were studied. Serotype of the isolates was confirmed using commercially available antisera. Except for arabinose fermentation, all the strains had similar biochemical reactions. More than 60% of the strains were sensitive to commonly used antibiotics; only 6% (n=7) of the strains were resistant to nalidixic acid, and none of the strains were resistant to mecillinam and ciprofloxacin. All strains were invasive as demonstrated by the presence of a 140 MDa plasmid, ial, sen and ipaH genes, Congo Red absorption ability and by the Sereny test performed on representative strains. Plasmid patterns were heterogeneous but more than 50% of strains were confined to a single pattern. All strains possessed a 1·6 MDa plasmid and 87% of the strains contained a 4 MDa plasmid. Middle-range plasmids (90 MDa to 30 MDa) present in 36% of the strains were not associated with antibiotic resistance. All the strains were clustered within a single type with four subtypes by pulsed-field gel electrophoresis while ribotyping patterns of all the strains were identical.
INTRODUCTION
Diarrhoeal disease is one of the leading causes of morbidity and mortality worldwide, and is ranked fourth as a cause of death [1] and second as a cause of years of productive life lost due to premature mortality and disability [2]. Shigellosis is one of the most important diarrhoeal diseases and is caused by members of the genus Shigella. Infection and outbreaks associated with this organism are prominent in developing countries and are strongly associated with overcrowding and poor hygienic conditions [3].
It has been estimated that more than 95 000 children <5 years of age die of shigellosis annually in Bangladesh [4]. One of the major problems in combating shigellosis is the increasing frequency of antibiotic resistance in Shigella spp. [5]. At present, 50% of Shigella are resistant to nalidixic acid in Bangladesh (surveillance data of ICDDR,B, 2002) and 29% in India [6]. Recent studies showed that third-generation cephalosporin- and fluoroquinolone-resistant strains of Shigella spp. pose an important threat in the treatment of dysentery especially in case of children [7, 8]. Rarely does susceptibility reappear once resistant strains have become endemic in a region. In order to ensure appropriate treatment, continual surveillance is required to determine which antibiotics are still active [9]. Therefore, the World Health Organization (WHO) has targeted Shigella as one of the enteric infections for which new vaccines are needed.
Immunity to Shigella is serotype specific and vaccine protection will, therefore, depend on the representation of each serotype in the vaccine [10, 11]. Hence, it is crucial to monitor the trends of the prevalence of serotypes, and within each predominant serotype the clonal distribution should be evaluated. In previous studies, we investigated the trends in the prevalence of S. flexneri and S. dysenteriae infections in Dhaka, Bangladesh [12, 13]. Dominant serotypes changed over time, as was also observed in India [6]. As an endemic zone, there is a huge burden of Shigella in Bangladesh with a variety of serotypes prevailing [12–15]. We attempted to characterize all the predominant serotypes of Shigella irrespective of the species. A large number of S. dysenteriae type 2 strains were isolated during the period 1999–2004 and the prevalence gradually increased over time; it was the most prevalent serotype among S. dysenteriae in 2002 (42%) [13]. In the present study, we have conducted a detailed characterization of S. dysenteriae type 2 isolates both at the phenotypic and genotypic level to determine the virulence factors and the clonal diversity among the strains.
MATERIALS AND METHODS
Bacterial strains
From January 1999 to December 2004, 113 strains of S. dysenteriae type 2 isolated from patients attending the Dhaka treatment centre operated by ICDDR,B were identified in the Clinical Microbiology Laboratory by using standard microbiological and biochemical methods [16]. The strains were grown in trypticase soy broth containing 0·3% yeast extract (TSBY) and stored at −70°C after addition of 15% glycerol. Identification at the serotype level was done by the slide agglutination test [12] using commercially available antisera kit (Denka Seiken, Tokyo, Japan). YSH6000, S. flexneri 2a [17] and an E. coli (ATCC 25922) strain that lacked the 140 MDa invasive plasmid and was sensitive to all antibiotics were used as positive and negative controls respectively, in the Sereny test and the test for Congo Red binding ability.
Biochemical characterization
The biochemical reactions of the strains were determined by standard methods [16].
Antimicrobial susceptibility
The susceptibilities of strains to antimicrobial agents were determined by the disc diffusion method, as recommended by the Clinical and Laboratory Standards Institute [18] using commercially available antimicrobial discs (Oxoid, Basingstoke, UK). The antibiotic discs used in this study were ampicillin (10 μg), mecillinam (25 μg), nalidixic acid (30 μg), sulphamethoxazole–trimethoprim (25 μg), and ciprofloxacin (5 μg). E. coli (ATCC 25922) and Staphylococcus aureus (ATCC 25923) were used as control strains for susceptibility tests.
Tests for invasiveness
To determine the invasive property, the strains were subjected to keratoconjunctivitis assay (Sereny test) and Congo Red absorption ability according to the procedures described previously [13, 15].
PCR assay
Detection of set1 gene (ShET-1), sen gene (ShET-2), ial gene, ipaH gene and stx genes was performed by amplifying set1A, set1B, sen, ial, ipaH and stx primers by PCR according to the procedures described previously [19]. All these primers were synthesized using Oligo 1000 DNA Synthesizer (Beckman, Fullerton, CA, USA) available in our laboratory at ICDDR,B.
Plasmid profile analysis
Plasmid DNA was prepared by the modified alkaline lysis method of Talukder et al. [14]. The molecular weight of the unknown plasmid DNA was assessed by comparing the mobilities of the plasmids of known molecular weights [20]. The plasmids present in the strains of E. coli PDK-9, R1, RP4, Sa and V517 as described previously [13] were used as molecular weight standards.
Determination of resistance factors
Conjugation experiments between multidrug resistant (AmpR, SxtR) strains, S. dysenteriae type 2 (K-727, K-1075) and the recipient E. coli K-12 (NalR, Lac+, F−) were performed by previously described method [21]. Transconjugant colonies were selected on MacConkey agar plates containing nalidixic acid (30 μg/ml) and ampicillin (50 μg/ml). Plasmid analysis and antimicrobial susceptibility testing of the transconjugants were performed to determine the transfer of plasmids with antibiotic resistance. Determinations of transfer frequency and curing of the resistance plasmid were done according to the method described earlier [21].
Pulsed-field gel electrophoresis (PFGE)
Intact agarose-embedded chromosomal DNA from S. dysenteriae type 2 strains were prepared and PFGE was performed using the contour-clamped homogeneous electric field apparatus (CHEF-DRII; Bio-Rad Laboratories, Richmond, CA, USA) according to the procedures described earlier [14, 22–24], but with different pulse times, 3–35 s for 8 h, 5–50 s for 10 h, 20–80 s for 10 h, and 60–120 s for 10 h. Genomic DNA was digested with NotI restriction enzyme (Gibco-BRL, Gaithersburg, MD, USA). The restriction fragments were resolved by using the CHEF-DRII system apparatus in 1% pulsed-field certified agarose in 0·5×TBE buffer; the gel was stained, de-stained, and photographed on a gel documentation system according to procedures described previously [14]. The DNA size standards used were the bacteriophage lambda ladder ranging from 48·5 kb to 1000 kb (Bio-Rad) and Saccharomyces cerevisiae chromosomal DNA ranging from 225 kb to 2200 kb (Bio-Rad). Band patterns were established by the criteria described previously [25].
Ribotyping
Ribotyping of S. dysenteriae type 2 strains was performed according to the procedure described previously [14]. In brief, chromosomal DNA was extracted, purified and digested with HindIII restriction enzyme for overnight at 37°C and separated by gel electrophoresis in 0·8% agarose in Tris borate EDTA (TBE) buffer according to the procedures described previously [14]. Southern blotting to a positively charged nylon membrane (Amersham Pharmacia Biotech, Buckinghamshire, UK) was performed with a vacuum pump unit (Bio-Rad) and the DNA fragments were fixed on the membrane by exposing to UV light for 3 min. Hybridization was performed with a digoxygenin (DIG)-labelled cDNA probe specific for the 16S ribosomal DNA (rDNA) (25) for 18 h at 42°C and development of the membrane with anti-DIG-alkaline phosphatase was performed according to the procedures described previously [15]. Results were documented by taking the photographs of the membrane in which the probe had hybridized with the resolved DNA fragments.
RESULTS
Biochemical characterization
A detailed biochemical study of representative strains of S. dysenteriae type 2 (n=42) showed that all the strains were positive for indole production and rhamnose fermentation within 48 h of incubation. Although, trehalose was fermented by all the strains, 22% showed a positive reaction within 48 h while the remaining strains (78%) fermented after 48 h. Arabinose was fermented by 29% of the strains of which 7% were positive within 48 h, while the remaining 22% strains fermented after 48 h. None of the strains could utilize sodium-acetate, xylose, raffinose, maltose, mannitol, dulcitol and sorbitol.
Antibiotic susceptibility
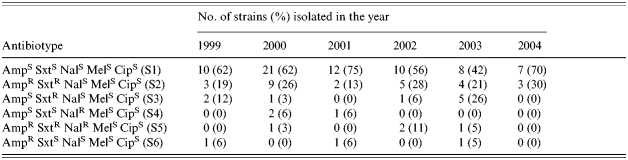
There were no consistent changes in antimicrobial sensitivity patterns observed over the time period studied. More than 60% of the strains were sensitive to all antibiotics examined in this study. During 1999, 25% were resistant to ampicillin (Amp), while, 29, 19, 39, 31 and 30% of the strains were resistant to Amp in the years 2000, 2001, 2002, 2003 and 2004 respectively. S. dysenteriae type 2 strains resistant to sulphamethoxazole–trimethoprim (Sxt) were 31% in 1999, 32% in 2000, 13% in 2001, 45% in 2002, 52% in 2003 and 30% in 2004. All the strains isolated in 1999 and 2004 were sensitive to nalidixic acid (Nal) while 9, 6, 11 and 5% of the strains were resistant to Nal in the years 2000, 2001, 2002 and 2003 respectively. All the strains of S. dysenteriae type 2 were sensitive to mecillinam and ciprofloxacin. Depending on the susceptibility results, the strains were grouped into six patterns (Table 1).
Table 1.
Antibiotic susceptibility pattern of S. dysenteriae type 2
Tests for invasiveness
Plasmid analysis showed that all the S. dysenteriae type 2 strains harboured the 140 MDa invasive plasmid (Table 2). All the strains (n=113) had the ability to absorb Congo Red dye. Five representative strains containing the 140 MDa plasmid were selected at random and were subjected to Sereny test. All the representative strains produced keratoconjunctivitis in a guinea pig's eye.
Table 2.
Plasmid profile analysis of S. dysenteriae serotype 2
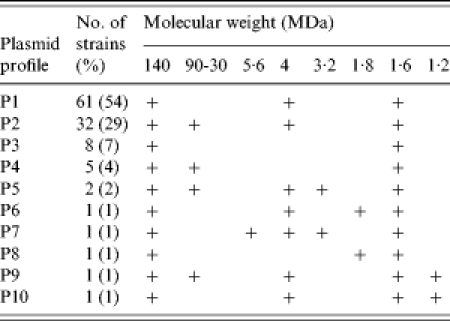
, Indicates the presence of plasmid.
PCR assays
Shigella enterotoxin 2 (sen), ial and ipaH genes were present in all the strains while Shigella enterotoxin 1 (set1) and stx genes were absent in all 113 strains of S. dysenteriae type 2.
Plasmid profile analysis
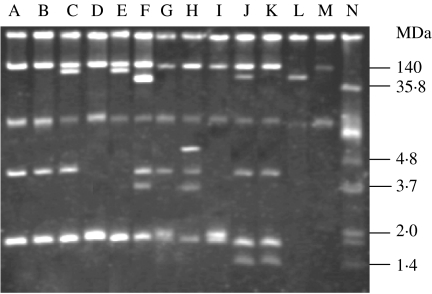
All the strains of S. dysenteriae type 2 contained multiple numbers of plasmids ranging from 140 to 1·0 MDa (Fig. 1). All the strains contained 140 MDa and 1·6 MDa plasmid, 89% of strains contained 4 MDa plasmid and 36% of strains harboured middle-range plasmids (90 MDa to 30 MDa). Plasmid patterns were created according to the number and size of the plasmid. Ten different patterns were found, of these, P1 (54%) was the predominant followed by P2 (29%), P3 (7%), P4 (4%), P5 (2%), P6 (1%), P7 (1%), P8 (1%), P9 (1%) and P10 (1%), these are described in detail in Table 2.
Fig. 1.
Agarose gel electrophoresis of plasmid DNA showing the representative patterns among the isolates of S. dysenteriae type 2. Lanes A, B, S. dysenteriae type 2 (pattern P1); lane C, S. dysenteriae type 2 (P2); lane D, S. dysenteriae type 2 (P3); lane E, S. dysenteriae type 2 (P4); lane F, S. dysenteriae type 2 (P5); lane G, S. dysenteriae type 2 (P6); lane H, S. dysenteriae type 2 (P7); lane I, S. dysenteriae type 2 (P8); lane J, S. dysenteriae type 2 (P9); lane K, S. dysenteriae type 2 (P10); lanes L, M, N, E. coli R-1, PDK-9, and V-517 respectively.
Determination of resistance factors
Antibiotic resistance pattern and plasmid profile analyses showed that 23% of the strains were resistant to ampicillin, and Sxt, of which 36% contained a middle-range plasmid having a molecular weight between 30 MDa and 90 MDa. Two strains designated as K-727 and K-1075 containing 62 MDa and 88 MDa respectively, having the antibiotic susceptibility pattern S2 (Table 1), were selected for conjugation experiments with E. coli K12. After conjugation, both the plasmids were transferred independently with the complete spectrum of drug resistance (AmpR SxtR). The transfer frequency was very high for all transmissible plasmids. All the transconjugants were cured by Acridine Orange losing the plasmids and became sensitive to all antibiotics (Table 3).
Table 3.
Transfer of resistance plasmid to E. coli K-12 by conjugation
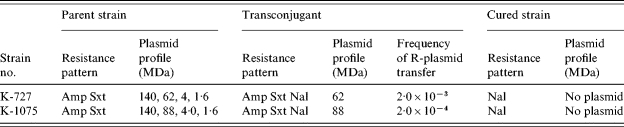
Amp, Ampicllin; Sxt, Sulphamethoxazole–trimethoprim; Nal, Nalidixic acid.
PFGE
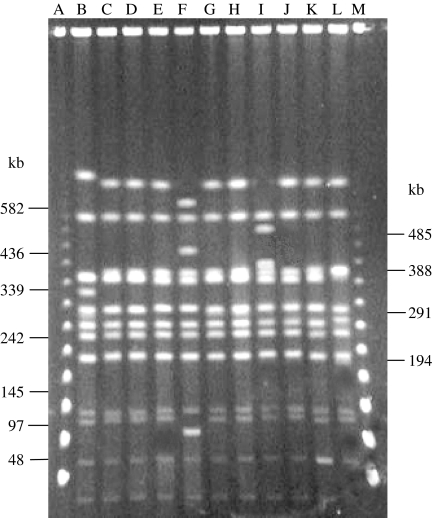
PFGE analysis of NotI-digested chromosomal DNA of S. dysenteriae type 2 strains yielded 12–14 reproducible DNA fragments ranging in size of ∼20 kb to 800 kb (Fig. 2). According to the criteria of interpretation for PFGE [25], all the strains were grouped into a single type (designated as A), which was further subdivided into four subtypes (A1–A4). Of these, A1 was the predominant pattern shared by 74% of the strains followed by A2 (18%), A3 (4%) and A4 (4%) (Fig. 2).
Fig. 2.
PFGE banding patterns of NotI-digested chromosomal DNA of representative strains of S. dysenteriae type 2. Lane A, λ ladder (marker); lanes B–L, S. dysenteriae type 2, K-467 (PFGE type A2); K-490 (A1); K-1075 (A1); K-1157 (A1); K-1455 (A3); K-1913 (A1); K-1141 (A1); K-1639 (A4); K-727 (A1); K-1822 (A1); K-1827 (A1) respectively; lane M, λ ladder (marker).
Ribotyping
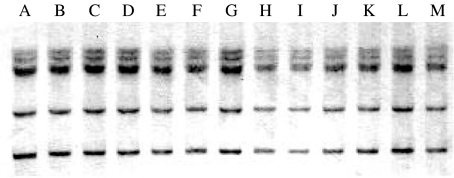
Hybridization of HindIII-digested chromosomal DNA of representative strains of S. dysenteriae type 2 with the 16S rDNA probe revealed a total of six fragments ranging in size of approximately 5–15 kb (Fig. 3). DNA fragments were arranged in a similar fashion in all the strains suggesting their identity in ribotyping pattern.
Fig. 3.
Ribotyping patterns of representative strains of S. dysenteriae type 2. Lanes A–M, S. dysenteriae type 2 (K-467, K-490, K-727, K-774, K-1075, K-1083, K-1141, K-1157, K-1158, K-1455, K-1639, K-1827 and KD-11), all strains showed same ribotype (R1).
DISCUSSION
As previously noted, the trend in prevalence of S. dysenteriae serotype has changed over the period 1999–2002. S. dysenteriae type 1, the most prevalent serotype in 1999 was replaced by serotypes of S. dysenteriae types 2 and 4 [13]. Recently, we have reported an unusual outbreak of S. dysenteriae type 4 that occurred between June and December 2000, in Dhaka, Bangladesh [26]. The prevalence of type 2 increased steadily from 1999 to 2002 [13], although major outbreaks caused by these type 2 strains have not yet been reported.
Our results suggest that S. dysenteriae type 2 strains in Bangladesh may be emerging from a common clone. Among the 22 biochemical tests carried out, only five reactions were positive of which, rhamnose, trehalose and glucose were utilized and indole was produced by all the strains within 48 h, however, extended incubation times were needed for 78% of the strains to ferment trehalose. Although limited responses were observed in biochemical reactions, all the strains exhibited the same reaction pattern, which matched the previous biochemical study [27]. Therefore, this biochemical reaction pattern can be used as a phenotypic marker for the preliminary identification of S. dysenteriae type 2 strains.
Antibiotic susceptibility pattern of strains isolated in different years did not show any significant variations. Although resistance to nalidixic acid was noted for strains isolated from 2000 to 2003, none of the strains isolated in 2004 were resistant to nalidixic acid. Additionally, among all the antibiotypes, S1 was predominant (>60%), which represented the strains sensitive to all antibiotics (Table 1). This is consistent with the limited antibiotic selection pressure put on strains that are circulating at a low level in the population [14].
Invasiveness is a key property of pathogenic Shigella strains, and is mainly conferred by a 140 MDa plasmid. However, the invasiveness genes on this plasmid are not fully operational without the activity of some chromosomal sequences. According to previous studies [28–32], it was observed that the virulence factor of Shigella is mainly conferred by a large plasmid of ∼140 MDa in which the key sequence of the invasive plasmid antigen is located, although it is not fully operative without the activity of some chromosomal sequences. All the strains in present study were actively virulent as is evident by the presence of the 140 MDa plasmid, IpaH, sen and ial genes and the ability to bind Congo Red and to produce keratoconjunctivitis in a guinea pig's eye.
In epidemiological studies, clonal distribution may be important to characterize for preventive treatment particularly in the early diagnosis and management of the disease. According to a previous report, plasmid profile analysis is well documented as meaningful in epidemiological studies of enteric pathogens [33]. It has been shown previously that S. dysenteriae type 2 strains possessed multiple numbers of plasmids with a heterogeneous pattern but three plasmids of 140, 4 and 1·6 MDa were commonly present in most of the strains designated as core plasmids [13]. Haider et al. [34] suggested that middle-range plasmids in Shigella are associated with antibiotic resistance with a self-transmissible property. In this study, 28% of strains were resistant to multiple drugs having a self-transmissible middle-range plasmid but at the same time 8% of strains were sensitive to all antibiotics carrying this plasmid. Therefore, it can be interpreted that all the middle-range plasmids are not associated with antibiotic resistance. Munshi et al. [21] reported that a 20 MDa plasmid was associated with nalidixic-acid resistance of S. dysenteriae type 1. In the present study, 6% (n=7) of S. dysenteriae type 2 strains were found to be resistant to nalidixic acid but none of the strains contained 20 MDa plasmid. Moreover, the strains did not harbour any middle-range plasmid, which concludes that nalidixic-acid resistance in S. dysenteriae type 2 strains is not transmissible via the plasmid.
PFGE and ribotyping have been considered to be highly discriminatory in subtyping strains of Shigella and other enteric pathogens. A single PFGE type was found in all the strains denoting their possible circulation from a single origin. However, little variation (2–6 bands) was observed in the banding patterns of the strains of single PFGE type, which grouped the strains into four subtypes, therefore characterizing them as possibly related clones. Additionally, there was no variation among strains observed by ribotyping.
The data presented here showed a high degree of conformance of the isolates by biochemical measures and ribotyping. Somewhat more variability was seen by PFGE and more was found in cases of antimicrobial sensitivity patterns and plasmid profiles. This strongly suggests a clonal expansion of the strains, with some diversification along the way. However, the variability being seen is occurring in characteristics that are most susceptible to change by the environmental influence or by local molecular events. It would be very interesting to compare the clones of S. dysenteriae type 2 in Bangladesh with the strains from other parts of the world in order to explain the epidemiological significance of this serotype in the global burden of shigellosis.
Acknowledgements
This study was funded by the United States Agency for International Development (USAID) under Cooperative Agreement No. HRN-A-00-96-90005-00 and ICDDR,B: Centre for Health and Population Research which is supported by countries and agencies which share its concern for the health problems of developing countries. Current donors providing unrestricted support include: the aid agencies of the Governments of Australia, Bangladesh, Belgium, Canada, Japan, The Netherlands, the Kingdom of Saudi Arabia, Sweden, Sri Lanka, Switzerland, the Bill and Melinda Gates Government of Bangladesh Fund, and the USA. ICDDR,B acknowledges with gratitude the commitment of USAID and other donors to the Centre's research effort.
DECLARATION OF INTEREST
None.
REFERENCES
- 1.Murray CJ, Lopez AD. Mortality by cause for eight reasons of the world: global burden of disease study. Lancet. 1997;349:1269–1276. doi: 10.1016/S0140-6736(96)07493-4. [DOI] [PubMed] [Google Scholar]
- 2.Murray CJ, Lopez AD. Global mortality, disability, and the contribution of risk factors: global burden of disease study. Lancet. 1997;349:1436–1442. doi: 10.1016/S0140-6736(96)07495-8. [DOI] [PubMed] [Google Scholar]
- 3.Melito PL et al. A novel Shigella dysenteriae serovar isolated in Canada. Journal of Clinical Microbiology. 2005;43:740–744. doi: 10.1128/JCM.43.2.740-744.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kotloff KL et al. Global burden of Shigella infections: implications for vaccine development and implementation of control strategies. Bulletin of the World Health Organization. 1999;77:651–666. [PMC free article] [PubMed] [Google Scholar]
- 5.Ashkenazi S et al. Growing antimicrobial resistance of Shigella isolates. Journal of Antimicrobial Chemotherapy. 2003;512:427–429. doi: 10.1093/jac/dkg080. [DOI] [PubMed] [Google Scholar]
- 6.Dutta S et al. Shifting serotypes, plasmid profile analysis and antimicrobial resistance pattern of shigellae strains isolated from Kolkata, India during 1995–2000. Epidemiology and Infection. 2002;129:235–243. doi: 10.1017/s0950268802007240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rahman M et al. Extended-spectrum beta-lactamase-mediated third-generation cephalosporin resistance in Shigella isolates in Bangladesh. Journal of Antimicrobial Chemotherapy. 2004;54:846–847. doi: 10.1093/jac/dkh413. [DOI] [PubMed] [Google Scholar]
- 8.Talukder KA et al. Genetic relatedness of ciprofloxacin-resistant Shigella dysenteriae type 1 strains isolated in south Asia. Journal of Antimicrobial Chemotherapy. 2004;54:730–734. doi: 10.1093/jac/dkh425. [DOI] [PubMed] [Google Scholar]
- 9.WHO. Antimicrobial resistance in shigellosis, cholera and campylobacteriosis. Geneva: World Health Organization; 2001. . Document WHO/CDS/CSR/DRS/8. [Google Scholar]
- 10.Fontaine A, Arondel J, Sansonetti PJ. Construction and evaluation of live attenuated vaccine strains of Shigella flexneri and Shigella dysenteriae 1. Research in Microbiology. 1990;141:907–912. doi: 10.1016/0923-2508(90)90129-e. [DOI] [PubMed] [Google Scholar]
- 11.Formal SB, Baker EE. Quantitative studies of cross-reactions between Shigella flexneri types 1a, 1b, and 3. Journal of Immunology. 1953;70:260–266. [PubMed] [Google Scholar]
- 12.Talukder KA et al. Altering trends in the dominance of Shigella flexneri serotypes and emergence of serologically atypical S. flexneri strains in Dhaka, Bangladesh. Journal of Clinical Microbiology. 2001;39:3757–3759. doi: 10.1128/JCM.39.10.3757-3759.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Talukder KA et al. Temporal shifts in the dominance of serotypes of Shigella dysenteriae from 1999 to 2002 in Dhaka, Bangladesh. Journal of Clinical Microbiology. 2003;41:5053–5058. doi: 10.1128/JCM.41.11.5053-5058.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Talukder KA et al. Phenotypic and genotypic characterization of serologically atypical strains of Shigella flexneri type 4 isolated in Dhaka, Bangladesh. Journal of Clinical Microbiology. 2002;40:2490–2497. doi: 10.1128/JCM.40.7.2490-2497.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Talukder KA et al. Phenotypic and genotypic characterization of provisional serotype Shigella flexneri 1c and clonal relationships with 1a and 1b strains isolated in Bangladesh. Journal of Clinical Microbiology. 2003;41:110–117. doi: 10.1128/JCM.41.1.110-117.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.WHO. Manual for laboratory investigations of acute enteric infections. Geneva: World Health Organization; 1987. . Document WHO/CDD/93.3 . [Google Scholar]
- 17.Sasakawa C et al. Molecular alteration of the 140-megadalton plasmid associated with loss of virulence and Congo red binding activity in Shigella flexneri. Infection and Immunity. 1986;51:470–475. doi: 10.1128/iai.51.2.470-475.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.National Committee for Clinical Laboratory Standards 14th. Villanova, PA: 2004. . Performance standards for antimicrobial susceptibility testing; approved standard, edn; document M100-S14. [Google Scholar]
- 19.Vargas M et al. Prevalence of Shigella enterotoxin 1 and 2 among Shigella strains isolated from patients with Traveler's diarrhea. Journal of Clinical Microbiology. 1999;37:3608–3611. doi: 10.1128/jcm.37.11.3608-3611.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Haider K et al. Electropherotyping of plasmid DNA of different serotypes of Shigella flexneri isolated in Bangladesh. Epidemiology and Infection. 1989;102:421–428. doi: 10.1017/s0950268800030132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Munshi MH et al. Plasmid mediated resistance to nalidixic acid in Shigella dysenteriae type 1. Lancet. 1987;2:419–421. doi: 10.1016/s0140-6736(87)90957-3. [DOI] [PubMed] [Google Scholar]
- 22.Talukder KA, Dutta DK, Albert MJ. Evaluation of pulsed-field gel electrophoresis for typing of Shigella dysenteriae type 1. Journal of Medical Microbiology. 1999;48:781–784. doi: 10.1099/00222615-48-8-781. [DOI] [PubMed] [Google Scholar]
- 23.Albert MJ et al. Phenotypic and genotypic changes in Vibrio cholerae O139 Bengal. Journal of Clinical Microbiology. 1997;35:2588–2592. doi: 10.1128/jcm.35.10.2588-2592.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Okada N et al. Construction of a physical map of the chromosome of Shigella flexneri 2a and the direct assignment of nine virulence-associated loci identified by Tn5 insertions. Molecular Microbiology. 1991;5:2171–2780. doi: 10.1111/j.1365-2958.1991.tb02147.x. [DOI] [PubMed] [Google Scholar]
- 25.Tenover FC et al. Interpreting chromosomal DNA restriction patterns produced by pulsed-field gel electrophoresis: criteria for bacterial strain typing. Journal of Clinical Microbiology. 1995;33:2233–2239. doi: 10.1128/jcm.33.9.2233-2239.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Talukder KA et al. An unusual cluster of dysentery due to Shigella dysenteriae type 4 in Dhaka, Bangladesh. Journal of Medical Microbiology. 2005;54:511–513. doi: 10.1099/jmm.0.45852-0. [DOI] [PubMed] [Google Scholar]
- 27.Ewing WH, Ewing WH. Identification of Enterobacteriaceae. New York: Elsevier Science Publishing Co. Inc; 1986. The genus Shigella; pp. 135–172. , pp. [Google Scholar]
- 28.Sansonetti PJ, Kopecko DJ, Formal SB. Involvement of a plasmid in the invasive ability of Shigella flexneri. Infection and Immunity. 1982;135:852–860. doi: 10.1128/iai.35.3.852-860.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sansonetti PJ et al. Alterations in the pathogenicity of Escherichia coli K-12 after transfer of plasmid and chromosomal genes from Shigella flexneri. Infection and Immunity. 1983;39:1392–1402. doi: 10.1128/iai.39.3.1392-1402.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Maurelli AT, Blackmon B, Curtiss R. Loss of pigmentation in Shigella flexneri is correlated with loss of virulence and virulence associated plasmid. Infection and Immunity. 1984;43:397–401. doi: 10.1128/iai.43.1.397-401.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Watanabe H, Nakamura A. Large plasmid associated with virulence in Shigella species have a common function necessary for epithelial cell penetration. Infection and Immunity. 1985;48:260–262. doi: 10.1128/iai.48.1.260-262.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Venkatesan MM, Buysse JM, Kopecko DJ. Use of Shigella flexneri ipaC and ipaH gene sequences for the general identification of Shigella spp. and enteroinvasive Escherichia coli. Journal of Clinical Microbiology. 1989;27:2687–2691. doi: 10.1128/jcm.27.12.2687-2691.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Surdeanu M et al. Differentiation of Shigella strains by plasmid profile analysis, serotyping and phage typing. Roumanian Archives of Microbiology and Immunology. 2000;59:103–117. [PubMed] [Google Scholar]
- 34.Haider K et al. Plasmid characterization of Shigella species isolated from children with shigellosis and asymptomatics excretors. Journal of Antimicrobial Chemotherapy. 1985;16:691–698. doi: 10.1093/jac/16.6.691. [DOI] [PubMed] [Google Scholar]