SUMMARY
Nasopharyngeal carriage of potential pathogens was studied in 425 healthy 3- to 6-year-old children attending 16 day-care centres (DCCs) in nine Czech cities during the winter 2004–2005. The overall carriage of pathogens was 62·8% (Streptococcus pneumoniae, 38·1%; Haemophilus influenzae, 24·9%; Moraxella catarrhalis, 22·1%; Staphylococcus aureus, 16%). An age-related downward trend was observed for colonization with respiratory pathogens in contrast to Staph. aureus whose carriage was significantly higher among older children. The following serotypes of colonizing S. pneumoniae were the most predominant: 23F (20·6%), 6A (15·1%), 6B (12·7%), 18C (7·8%), 15B and 19F (6% each). The majority (94·3%) of H. influenzae isolates were non-typable; among capsulated isolates, serotype b was not found. Decreased susceptibility to penicillin was determined in 3% of pneumococci; 4·6% of H. influenzae strains and 85·1% of M. catarrhalis strains produced β-lactamase. As for non-β-lactam antibiotics, pneumococci resistant to trimethoprim–sulphamethoxazole were the most common (15·7%) among the attendees.
INTRODUCTION
Several potential pathogenic bacterial species such as Streptococcus pneumoniae, Haemophilus influenzae, Moraxella catarrhalis, Staphylococcus aureus, and Neisseria meningitidis are often part of the resident flora especially in children. Although usually asymptomatic, carriage of these microorganisms in the upper respiratory tract can occasionally progress into respiratory or systemic disease [1, 2]. The carriage state is, therefore, a potential source for the horizontal spread of these organisms in the community [3].
Children attending day-care centres (DCCs) are an excellent reservoir for the transmission of bacterial pathogens due to crowding and their age [3, 4]. Evaluation of the prevalence of bacterial carriage among children together with determination of antimicrobial susceptibility of strains can provide useful information on antibiotic resistance among bacteria circulating in the community as well as the efficacy of present or future immunization against these organisms. In the Czech Republic, antibiotic resistance rates among respiratory pathogens in young children remains low [5–7]. A periodical nationwide surveillance study of antibiotic susceptibility among respiratory pathogens from sputum samples collected from October to December 2004 revealed a prevalence of only 3·1% of penicillin non-susceptible S. pneumoniae (PNSP) strains [8] and 6·1% of β-lactamase-positive H. influenzae strains in children <7 years old. The overall low level of resistance to antibiotics is historically attributed to a more restrictive use of antibiotics together with the preference of general practitioners for narrow-spectrum penicillins for treatment. The current standards for treatment of community-aquired respiratory infections in primary care, approved by the Czech Medical Association of J. E. Purkyně [9], recommend amoxicillin as the drug of choice for treatment of respiratory infection caused by S. pneumoniae and H. influenzae on the basis of a low prevalence of resistance to β-lactam antibiotics in both species in the Czech Republic.
Compulsory vaccination of children <1 year old against Hib was introduced in the Czech Republic in July 2001. A nationwide surveillance programme, after the introduction of routine vaccination, showed that morbidity due to Hib invasive disease in the targeted group of children dropped from 15·6 cases/100 000 population in 2001 to 3·3 cases/100 000 population in 2003 [10]. A pneumococcal 7-valent conjugate vaccine has been available in the Czech Republic since September 2005 and currently, vaccination is carried out upon the request of the child's parents or upon the recommendation of his/her paediatrician, and is paid for by the parents.
The aim of this study was to obtain data on the carriage of respiratory pathogens, i.e. S. pneumoniae, H. influenzae and M. catarrhalis, and to determine the prevalence of resistance to antibiotics among nasopharyngeal isolates in preschool children attending DCCs in the Czech Republic during the winter season 2004–2005. Data on the occurence and serotype distribution of S. pneumoniae and H. influenzae in addition to the frequency of Staph. aureus and antibiotic susceptibility in the nasopharyngeal flora of these children are presented. The influence of age, gender, and bacterial co-colonization on the carrier status was also investigated.
MATERIALS AND METHODS
Sampling
Children attending 16 DCCs in nine Czech cities were studied from December 2004 to April 2005. The study cities were designated with capital letters as follows: A, Prague; BB, Brno; CB, České Budějovice; HK, Hradec Králové; JH, Jindřichův Hradec; MO, Most; OL, Olomouc; OV, Ostrava; PL, Plzeň. A signed informed consent form was obtained from each participant's parents. Samples of the nasopharyngeal flora were obtained pernasally using synthetic cotton swabs on flexible aluminium wire (Venturi Transystem®, Copan Italia Spa, Brescia, Italy). The swabs were transported within 2–5 h from collection in Amies transport medium without charcoal to the National Institute of Public Health (Prague) to be processed immediately. They were cultured on (i) Columbia agar with 5% sheep's blood, (ii) Columbia agar with 5% sheep's blood and gentamicin (4 mg/l) and (iii) Levinthal's agar. The plates were incubated at 36°C in 5% CO2-enriched atmosphere for up to 48 h. Identification was based on the following conventional microbiological methods: colony morphology, growth on Columbia agar with gentamicin, susceptibility to optochin (Oxoid, Basingstoke, Hants, UK), and bile solubility for S. pneumoniae; colony morphology, growth on Levinthal's agar, X+V factor requirements (positive porphyrin test for factor X, satellitism around Staph. aureus for factor V) for H. influenzae; colony morphology, positive oxidase (PLIVA Lachema, Brno, Czech Republic) and DNase tests for M. catarrhalis, and colony morphology and positive tube coagulase test (ITEST Plus Ltd, Hradec Králové, Czech Republic) for Staph. aureus. For S. pneumoniae, any differencies in colony morphology within a patient's culture were further analysed by serotyping and susceptibility testing.
Serotyping
Pneumococci were serotyped by the Quellung reaction using type and factor sera provided by the Statens Institut (Copenhagen, Denmark) [11]. H. influenzae strains were serotyped by a slide agglutination test using six monovalent antisera (serotypes a–f) manufactured by Remel (Remel Inc., Lenexa, KS, USA).
Antibiotic susceptibility testing
Minimum inhibitory concentrations (MICs) of antimicrobial agents were determined by the broth microdilution method as described by the Clinical and Laboratory Standards Institute (CLSI, formerly the National Committee for Clinical Laboratory Standards) [12]. S. pneumoniae was tested for susceptibility to penicillin, amoxicillin, cefotaxime, erythromycin, clindamycin, chloramphenicol, tetracycline, trimethoprim–sulfamethoxazole (TMP–SMX), and rifampin; H. influenzae was tested for susceptibility to amoxicillin, amoxicillin–clavulanic acid, cefuroxime, cefotaxime, chloramphenicol, tetracycline, TMP–SMX, ciprofloxacin, and rifampin; Staph. aureus was tested for susceptibility to oxacillin, erythromycin, clindamycin, chloramphenicol, tetracycline, TMP–SMX, rifampin, gentamicin, ciprofloxacin, and vancomycin; and M. catarrhalis was tested for susceptibility to amoxicillin, cefotaxime, erythromycine, tetracycline, chloramphenicol, and TMP–SMX. Standard reference strains recommended by CLSI were included for quality control. The test concentrations of antibiotics were prepared using ADATABTM tablets (Mast Group Ltd, Bootle, Merseyside, UK). For H. influenzae, M. catarrhalis and Staph. aureus isolates, the production of β-lactamases was tested by the nitrocefin method (Oxoid). The CLSI standards were applied to interpret MIC results but intermediate resistant strains were considered as resistant [12, 13].
Statistical analysis
The Epi-Info 3.3.2 statistical package (CDC, Atlanta, GA, USA) was used to test the differences in distribution between the studied populations. The crude odds ratio (OR) and Mantel–Haenszel OR stratified by age with 95% confidence intervals (CIs) were calculated using χ2 test. A P value of <0·05 was considered significant.
RESULTS
Study population
A total of 425 children (218 boys) aged from 3 to 6 years were enrolled in the study. The number of sampled children per participating DCC ranged from 10 to 69 and the number of sampled children per participating city ranged from 14 to 98. The available vaccination data were incomplete. As routine vaccination against H. influenzae type b (Hib) was introduced for children <1 year old from July 2001, children born after 31 July 2000 (n=159) were assumed to have been Hib vaccinated. At the time of sampling, the pneumococcal conjugate vaccine was not available for clinical use in the Czech Republic.
Nasopharyngeal colonization
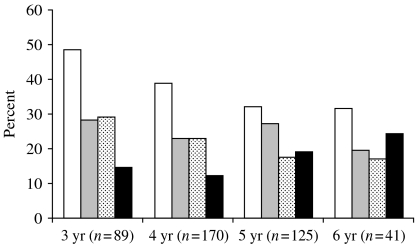
The overall carriage rates of pathogenic bacteria were 38·1% (n=162) for S. pneumoniae, 24·9% (n=106) for H. influenzae, 22·1% (n=94) for M. catarrhalis and 16% (n=68) for Staph. aureus. A total of 165 S. pneumoniae strains were isolated, as three children carried two strains of different serotypes. Only single isolates of H. parainfluenzae and Streptococcus pyogenes were identified. Younger age (⩽4 years) was significantly associated with S. pneumoniae colonization (OR 1·55, 95% CI 1·01–2·38, P=0·04) while Staph. aureus colonization was significantly more frequent (OR 0·59, 95% CI 0·35–0·99, P=0·04) among older children (>4 years). The carriage of H. influenzae and M. catarrhalis declined with age, but no significant association was found (P=0·89 and 0·06 respectively; Fig.). S. pneumoniae was the most frequently carried pathogen regardless of age. No significant association was found between gender and colonization by a specific bacterial species with the exception of H. influenzae which was significantly more frequent among boys: 64 boys vs. 42 girls were colonized (OR 0·61, 95% CI 0·38–0·98, P=0·03). The major difference in the proportions of H. influenzae colonization between genders was observed in 4-year-olds (25 boys vs. 14 girls were colonized). When influence of age was eliminated using the Mantel–Haenszel test, the significance of connection between the incidence of H. influenzae colonization and male sex was limited (OR 0·63, 95% CI 0·39–1, P=0·05).
Fig.
Recovery of specific pathogens in the nasopharynx by age. □, S. pneumoniae; , H. influenzae;
, H. influenzae; , M. catarrhalis; ■, Staph. aureus.
, M. catarrhalis; ■, Staph. aureus.
The overall carriage of respiratory pathogens was 62·8% (n=267). Almost half of children (n=183, 43%) carried only one respiratory pathogen. A total of 84 children were colonized by more than one pathogen. Seventy-three children (17·1%) carried two respiratory pathogens and 11 children (2·5%) carried three species (Table 1). In 32 (7·5%) children, respiratory pathogens were present in combination with either Staph. aureus (n=31, 7·2%) or S. pyogenes in one child. A negative correlation (OR 0·44, 95% CI 0·23–0·84, P=0·006) was noted for colonization with pneumococci and Staph. aureus (n=16, 16% of co-colonization) compared with cultures negative for pneumococci (n=52, 76·4%). No significant associations were observed between S. pneumoniae and H. influenzae, S. pneumoniae and M. catarrhalis, and Staph. aureus and H. influenzae co-colonization.
Table 1.
Recovery of respiratory pathogens from nasopharyngeal cultures of children according to age group
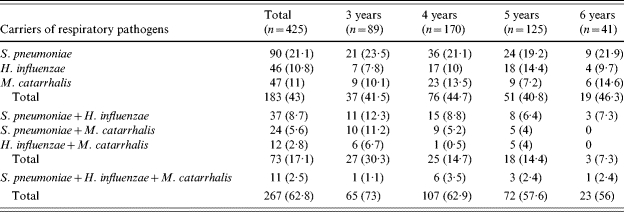
Numbers in parentheses are percentages.
Serotyping
Altogether 165 S. pneumoniae isolates and 106 H. influenzae isolates were serotyped. Among S. pneumoniae, as many as 21 serotypes were recognized within 16 serogroups and their distribution by cities is shown in Table 2. Serogroup 6 was the most prevalent, and 81% of all strains belonged to serogroups 6, 23, 15, 18, 19, and 11 in descending order. Although all colonies showing different morphologies were serotyped separately, only three cases of multiple serotype carriage were confirmed. Three children carried two isolates of different serotypes, i.e. 3+6B (n=2), and 3+15B. All of the serotypes covered by the 7-valent pneumococcal conjugate vaccine, i.e. 4, 6B, 9V, 14, 18C, 19F, and 23F, were found among the nasopharyngeal isolates. However, the proportions of individual serotypes varied widely, ranging from 1 (0·6%) isolate of serotype 9V to 34 (20·6%) isolates of serotype 23F. The theoretical vaccine coverage of the 7-valent conjugate vaccine for the serotypes identified is 51·5% (n=85). Six (5·6%) of 106 H. influenzae isolates were capsulated and capsular serotypes e and f were represented by three isolates each. Isolates of serotype e were recovered in the same DCC (OV, Ostrava), and two isolates of serotype f were also found together in one DCC, but in a different city (CB, České Budějovice). The remainder of the H. influenzae isolates were non- capsulated.
Table 2.
Distribution of S. pneumoniae serotypes by city
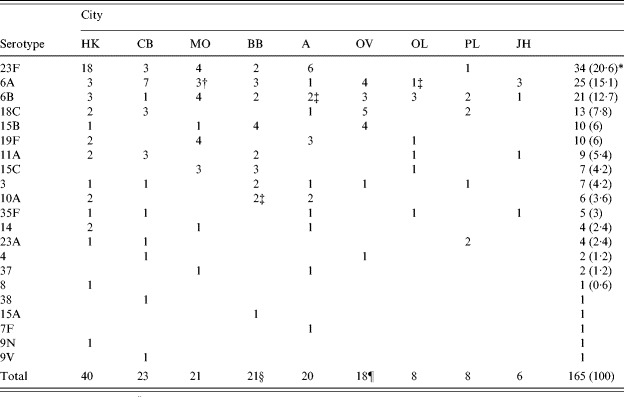
HK, Hradec Králové; CB, České Budějovice; MO, Most; BB, Brno; A, Prague; OV, Ostrava; OL, Olomouc; PL, Plzeň; JH, Jindřichův Hradec.
Numbers in parentheses are percentages.
Two penicillin-intermediary resistant strains, MIC 0·125 mg/l.
One penicillin-intermediary resistant strain, MIC 0·125 mg/l.
Two children harboured two isolates of different serotypes, i.e. 3+6B and 3+15B.
One child harboured two isolates of different serotypes, i.e. 3+6B.
Resistance to antimicrobial agents
Altogether isolates of the four species were tested for resistance to antibiotics. List of antibiotics and frequency of resistance are shown in Table 3. Table 3 shows five (3%) PNSP with an MIC of 0·125 mg/l. These strains fell into serotypes 6A, 6B and 10A (Table 2). Four PNSP strains were susceptible to other tested antibiotics while a single PNSP strain of serotype 10A was concomitantly resistant to erythromycin, clindamycin, tetracycline, and TMP–SMX. Resistance to certain antibiotics was predominantly associated with serogroup/serotype. All but one of 18 isolates of serogroup 15 were resistant to TMP–SMX and eight of nine serotype 19F strains were resistant to chloramphenicol; six were also tetracycline resistant. Resistance to amoxicillin was found in five (4·6%) H. influenzae strains and in 80 (85·1%) M. catarrhalis isolates, all of these produced β-lactamase. All β-lactamase-positive H. influenzae strains were non-typable. Sixty-two (88·5%) Staph. aureus isolates were resistant to penicillin due to β-lactamase production; resistance to oxacillin was not detected.
Table 3.
Resistance to antimicrobial agents of S. pneumoniae, H. influenzae, M. catarrhalis and S. aureus from the nasopharynx of children
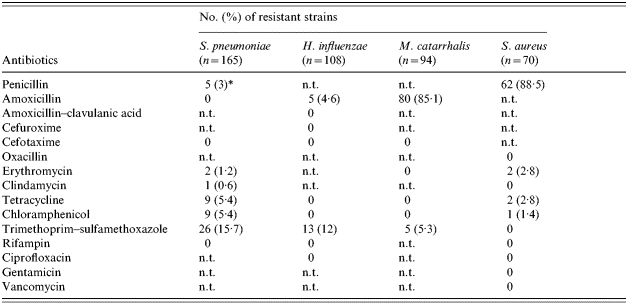
n.t., Not tested.
Penicillin-intermediate resistant strains, MIC 0·125 mg/l.
Discussion
During the first year of life, the nasopharynx of infants becomes colonized by a variety of microorganisms, including commensal bacteria as well as potential pathogens [1, 14]. Since the colonization is markedly influenced by numerous socioeconomic, environmental and host-related factors, the reported rates of carriage vary widely with different studies and geographical sites [4, 15]. The objective of the present study was to establish the prevalence and certain features of S. pneumoniae, H. influenzae, M. catarrhalis, and Staph. aureus strains presently circulating among DCC attendees in the Czech Republic.
In our study, a major group of children (62·8%) harboured at least one potential respiratory pathogen. The overall carriage of pathogens except for H. influenzae was comparable to that found in other European countries such as The Netherlands, Sweden, Poland and Greece [16–21]. H. influenzae carriage (24·9%) was lower than the carriage rates of 35%, 37% and 54·8% reported in Sweden, The Netherlands and Spain respectively [18, 19, 22]. Possible explanations of this difference may be the older age of children in our study and/or the relatively high incidence of previous upper respiratory tract infections among sampled children in the Dutch study. A previous study carried out in the Czech Republic from June 1993 to October 1994 showed 12% of children to be colonized with S. pneumoniae [23] which is in marked contrast to the pneumococcal carriage rate of 38·1% found here. The use of a different methodology is most likely to be the reason for this dissimilarity rather than the discrepancy in season of sampling, as respiratory pathogens can be recovered from the nasopharynx of healthy children all year round [24, 25], although some authors [26, 27] reported that the rates of colonization tend to be seasonal.
After peaking at 2–3 years of life, colonization with respiratory pathogens decreases with age [17, 25]. We also observed an age-related decline in colonization with respiratory pathogens, but the difference in the carriage rates between young and older children was statistically significant for pneumococcal colonization only. The decline in pneumococcal colonization was accompanied by a simultaneous increase in the carriage of Staph. aureus from 14·6% in 3-year-olds to 24·3% at 6 years of age. The Dutch study focused on dynamics of nasopharyngeal colonization with S. pneumoniae and Staph. aureus in a large cohort of healthy children and adolescents aged 1–18 years. The peak incidence of pneumococci was recorded in 3-year-olds, followed by a gradual decline until reaching a stable colonization rate after the age of 10 years [17]. A negative correlation was noted for co-colonization by Staph. aureus and vaccine type pneumococci, but not for Staph. aureus and non-vaccine type pneumococci. As in our study, the total number of children harbouring both of these pathogens was very low (16 children); unfortunately, a subsidiary analysis of this correlation for groups of vaccine and non-vaccine pneumococci was not performed.
The serotype distribution of colonizing S. pneumoniae strains provides background information for prediction of serotype-specific invasive disease potential: the calculation of invasiveness is based on comparison of the prevalence of each serotype among invasive and carriage isolates [28]. The knowledge of invasiveness of non-vaccine serotypes is crucial as it helps to estimate their possible effect on invasive disease after vaccination is widely distributed. In our set of pneumococci, the most predominant colonizing serotypes were 23F (20·6%), 6A (15·1%), 6B (12·7%), 18C (7·8%), 15B and 19F (6% each). This is in accordance with the previously reported data on pneumococcal colonization, showing that serogroups 6, 19, and 23 are the most common [14, 16, 20, 21, 29, 30]. With the exception of 15B, all the above-mentioned serotypes and serotype 1 were also predominant among invasive isolates recovered from children (n=168) in the Czech Republic from 1996 to 2003 [31]. Continuous surveillance of pneumococcal serotypes will be more important when wider pneumococcal prophylaxis is introduced in the Czech Republic as it will help to trace the effect of vaccine use on the pneumococcal population.
The prevalence of H. influenzae serotype b (Hib) carriers shows high variability in both the vaccinated and unvaccinated populations [20, 32–34]. For example in a Catalonian study [22], the prevalence of Hib carriage was very low and almost the same in vaccinated (1%) and unvaccinated (0·9%) preschool children. Introduction of Hib vaccination led to a significant reduction of Hib disease and carriage in both vaccinated and unvaccinated children due to a herd immune effect [33]. Although more than 60% of children in our study were assumed not to be vaccinated against Hib, surprisingly, no Hib strain was recovered. This may have been due to high immune status of the Czech population, as shown by the 2001 serological survey prior to the introduction of compulsory vaccination against Hib. The total anti-Hib antibody level needed for long-term protection (1 μg/ml) was detected in 99% of screened sera from unvaccinated persons (n=2479) of all age groups [35]. Nevertheless, no data are available on the prevalence of Hib carriage before the routine vaccination was implemented and the absence of Hib isolates is likely to be a result of a very low prevalence of Hib carriage alone, rather than of a herd immune effect following immunization.
The prevalence rates of β-lactam-resistant carriage isolates found in our study are slightly lower than those recorded for invasive strains collected from children, with 5·0% of pneumococci isolated between 2000 and 2004 having reduced susceptibility to penicillin [8] and 4·8% of non-typable H. influenzae strains isolated between 1999 and 2002 producing β-lactamase [7]. Our results support the assumption that the survey of resistance to antibiotics among nasopharyngeal isolates would be helpful in predicting resistance rates among invasive strains in the community [36, 37].
As for non-β-lactam antibiotics, pneumococci resistant to TMP–SMX were most common in the attendees. This is in accordance with the results of other studies that also found the highest rates of resistance to TMP–SMX in pneumococci, ranging from 24 to 35%, frequently with co-resistance to penicillin [14, 38, 39]. Once more, the highest rates of resistance to chloramphenicol and tetracycline (5·4% for each) were found in pneumococci. These findings are surprising as chloramphenicol and tetracycline are only rarely prescribed to children in the Czech Republic. A study from the United Kingdom demonstrated that healthy children may be colonized by strains of different species with acquired resistance to antibiotics (ceftazidime, chloramphenicol, and tetracycline) without previous exposure to such therapeutic agents [40]. The authors hypothesized that resistance to these antibiotics may be a result of co-selection due to exposure to other antibiotics used in children or may be acquired through close family contacts or food. In our study, resistance to TMP–SMX or chloramphenicol and tetracycline was associated with serotypes, 15B/C and 19F respectively. This observation is consistent with results of other studies showing that the spread of particular resistant clones had a remarkable contribution to the circulation of antibiotic-resistant strains in the community [14, 16, 18–21 14, 16, 18–21, 39, 41].
The survey of nasopharyngeal colonization provides data on important characteristics of specific microorganisms prevalent in the studied population and on the status of resistance to antibiotics which are helpful in designing appropriate empirical antimicrobial therapy. Continuous surveillance of carriage will allow us to establish the effect of conjugate vaccines use on S. pneumoniae and H. influenzae serotype distribution and to evaluate the influence of vaccination on the natural balance of the resident flora and its consequences.
Acknowledgements
We thank Tamara Bergerová, Dagmar Burgetová, Ljuba Čekanová, Markéta Hanslianová, Blanka Heinigeová, Magdalena Horníková, Eva Chmelařová, Věra Toršová, Milan Kolář, Miriam Koupilová, Evženie Pozlerová, Marie Mlynaříková, and Roman Záruba from regional microbiology laboratories for providing contacts in local DCCs. This study was partly supported by contract LSHM-CT-2003-503413 (Pneumococcal Resistance Epidemicity and Virulence – an International Study, PREVIS), from the European Commission.
DECLARATION OF INTEREST
None.
References
- 1.Faden H et al. Relationship between nasopharyngeal colonization and the development of otitis media in children. Journal of Infectious Diseases. 1997;175:1440–1445. doi: 10.1086/516477. [DOI] [PubMed] [Google Scholar]
- 2.Obaro S, Adegbola R. The pneumococcus: carriage, disease and conjugate vaccines. Journal of Medical Microbiology. 2002;51:98–104. doi: 10.1099/0022-1317-51-2-98. [DOI] [PubMed] [Google Scholar]
- 3.Leiberman A et al. The bacteriology of the nasopharynx in childhood. International Journal of Pediatic Otorhinolaryngology. 1999;49:151–153. doi: 10.1016/s0165-5876(99)00151-2. (Suppl 1): [DOI] [PubMed] [Google Scholar]
- 4.Garcia-Rodriguez JA, Fresnadillo Martinez MJ. Dynamics of nasopharyngeal colonization by potential respiratory pathogens. Journal of Antimicrobial Chemotherapy. 2002;50:59–73. doi: 10.1093/jac/dkf506. (Suppl 2): [DOI] [PubMed] [Google Scholar]
- 5.Urbášková P, Motlová J. Occurrence of Streptococcus pneumoniae resistant to penicillin and other antibiotics in the Czech Republic from 1996 to 1998 [in Czech] Klinická mikrobiologie a infekční lékařství. 1999;5:65–71. [Google Scholar]
- 6.Urbášková P, Motlová J, Žemličková H. Antibiotic resistance in invasive pneumococci and their serotypes in the Czech Republic [in Czech] Časopis Lékařů Českých. 2004;143:178–183. [PubMed] [Google Scholar]
- 7.Urbášková P et al. Resistance to ten antibiotics in invasive isolates of Haemophilus influenzae isolated in the Czech Republic over the period of 1999–2002 [in Czech] General Practitioner. 2004;54:705–709. [Google Scholar]
- 8.Urbášková P. Trends in penicillin and erythromycin resistance among pneumococci isolated from children with respiratory and/or invasive infection [in Czech] Bulletin of the Centre of Epidemiology and Microbiology. 2005;14:440–443. [Google Scholar]
- 9.Bébrová E et al. Recommended procedure for antimibrobial therapy of community respiratory infections in primary care [in Czech] General Practitioner. 2003;83:502–515. [Google Scholar]
- 10.Křížová P, Lebedová V, Beneš Č. The effect of routine vaccination in the Czech Republic on the incidence of invasive diseases caused by Haemophilus influenzae b [in Czech] Klinická mikrobiologie a infekční lékařství. 2004;10:118–123. [PubMed] [Google Scholar]
- 11.Sorensen UB. Typing of pneumococci by using 12 pooled antisera. Journal of Clinical Microbiology. 1993;31:2097–2100. doi: 10.1128/jcm.31.8.2097-2100.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.National Committee for Clinical Laboratory Standards (NCCLS) 6th. 2003. . Document M07-A6. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically; approved standard ( edn),
- 13.National Committee for Clinical Laboratory Standards (NCCLS) 2004. . Document M100-S14. Performance standards for antimicrobial susceptibility testing; 14th informational Supplement,
- 14.Sluijter M et al. Molecular characterization of pneumococcal nasopharynx isolates collected from children during their first 2 years of life. Journal of Clinical Microbiology. 1998;36:2248–2253. doi: 10.1128/jcm.36.8.2248-2253.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bogaert D, de Groot R, Hermans PWM. Streptococcus pneumoniae colonisation: the key to pneumococcal disease. Lancet Infectious Diseases. 2004;4:144–154. doi: 10.1016/S1473-3099(04)00938-7. [DOI] [PubMed] [Google Scholar]
- 16.Bogaert D et al. Pneumococcal carriage in children in the Netherlands: a molecular epidemiological study. Journal of Clinical Microbiology. 2001;39:3316–3320. doi: 10.1128/JCM.39.9.3316-3320.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bogaert D et al. Colonisation by Streptococcus pneumoniae and Staphylococcus aureus in healthy children. Lancet. 2004;363:1871–1872. doi: 10.1016/S0140-6736(04)16357-5. [DOI] [PubMed] [Google Scholar]
- 18.Peerbooms PGH et al. Nasopharyngeal carriage of potential bacterial pathogens related to day care attendance, with special reference to the molecular epidemiology of Haemophilus influenzae. Journal of Clinical Microbiology. 2002;40:2832–2836. doi: 10.1128/JCM.40.8.2832-2836.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Henriques Normark B et al. Clonal analysis of Streptococcus pneumoniae nonsusceptible to penicillin at day-care centers with index cases, in a region with low incidence of resistance: emergence of an invasive type 35B clone among carriers. Microbial Drug Resistance. 2003;9:337–344. doi: 10.1089/107662903322762761. [DOI] [PubMed] [Google Scholar]
- 20.Sulikowska A et al. Characteristics of Streptococcus pneumoniae, Haemophilus influenzae, and Moraxella catarrhalis isolated from the nasopharynges of asymptomatic children and molecular analysis of S. pneumoniae and H. influenzae strain replacement in the nasopharynx. Journal of Clinical Microbiology. 2004;42:3942–3949. doi: 10.1128/JCM.42.9.3942-3949.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tsolia M et al. Prevalence and patterns of resistance of Streptococcus pneumoniae strains isolated from carriers attending day care centers in the area of Athens. Microbial Drug Resistance. 1999;5:271–278. doi: 10.1089/mdr.1999.5.271. [DOI] [PubMed] [Google Scholar]
- 22.Fontanals D et al. Prevalence of Haemophilus influenzae carriers in the Catalan preschool population. European Journal of Clinical Microbiology and Infectious Diseases. 2000;19:301–304. doi: 10.1007/s100960050480. [DOI] [PubMed] [Google Scholar]
- 23.Appelbaum PC et al. Carriage of antibiotic-resistant Streptococcus pneumoniae by children in Eastern and Central Europe – a multicenter study with use of standardized methods. Clinical Infectious Diseases. 1996;23:172–177. doi: 10.1093/clinids/23.4.712. [DOI] [PubMed] [Google Scholar]
- 24.Marchissio P et al. Seasonal variations in nasopharyngeal carriage of respiratory pathogens in healthy Italian children attending day-care centres or schools. Journal of Medical Microbiology. 2001;50:1095–1099. doi: 10.1099/0022-1317-50-12-1095. [DOI] [PubMed] [Google Scholar]
- 25.Syrjanen RK et al. Nasopharyngeal carriage of Streptococcus pneumoniae in Finnish children younger than 2 years old. Journal of Infectious Diseases. 2001;184:451–459. doi: 10.1086/322048. [DOI] [PubMed] [Google Scholar]
- 26.Van Hare GF et al. Acute otitis media caused by Branhamella catarrhalis: biology and therapy. Reviews of Infectious Diseases. 1987;9:16–27. doi: 10.1093/clinids/9.1.16. [DOI] [PubMed] [Google Scholar]
- 27.Yagupsky P et al. Acquisition, carriage, and transmission of pneumococci with decreased antibiotic susceptibility in young children attending a day care facility in southern Israel. Journal of Infectious Diseases. 1998;177:1003–1012. doi: 10.1086/515239. [DOI] [PubMed] [Google Scholar]
- 28.Brueggemann AB et al. Clonal relationships between invasive and carriage Streptococcus pneumoniae and serotype- and clone-specific differences in invasive disease potential. Journal of Infectious Diseases. 2003;187:1124–1132. doi: 10.1086/374624. [DOI] [PubMed] [Google Scholar]
- 29.Meats E et al. Stability of serotypes during nasopharyngeal carriage of Streptococcus pneumoniae. Journal of Clinical Microbiology. 2003;41:386–392. doi: 10.1128/JCM.41.1.386-392.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sá-Leão R et al. Genetic diversity and clonal patterns among antibiotic-susceptible and -resistant Streptococcus pneumoniae colonizing children: day care centers as autonomous epidemiological units. Journal of Clinical Microbiology. 2000;38:4137–4144. doi: 10.1128/jcm.38.11.4137-4144.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Motlová J. Distribution of Streptococcus pneumoniae serotypes and serogroups among patients with invasive pneumococcal diseases in the Czech Republic in 1996–2003: Background data for vaccination strategy [in Czech] Epidemiologie, Mikrobiologie, Imunologie. 2005;54:3–10. [PubMed] [Google Scholar]
- 32.Faden H et al. Epidemiology of nasopharyngeal colonization with non-typeable Haemophilus influenzae in the first two years of life. Journal of Infectious Diseases. 1995;172:132–135. doi: 10.1093/infdis/172.1.132. [DOI] [PubMed] [Google Scholar]
- 33.Heath PT, McVernon J. The UK Hib vaccine experience. Archives of Disease in Childhood. 2002;86:396–399. doi: 10.1136/adc.86.6.396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Galil K et al. Reemergence of invasive Haemophilus influenzae type b disease in a well-vaccinated population in remote Alaska. Journal of Infectious Diseases. 1999;179:101–106. doi: 10.1086/314569. [DOI] [PubMed] [Google Scholar]
- 35.Lebedová V, Křížová P. The 2001 serological survey in the Czech Republic – Hib invasive disease Haemophilus influenzae b. Central European Journal of Public Health. 2003;11:25–30. [PubMed] [Google Scholar]
- 36.Kellner JD et al. The use of Streptococcus pneumoniae nasopharyngeal isolates from healthy children to predict features of invasive disease. Pediatric Infectious Disease Journal. 1998;17:279–286. doi: 10.1097/00006454-199804000-00004. [DOI] [PubMed] [Google Scholar]
- 37.Kouppari G et al. Serotyping and antibiotic resistance of Streptococcus pneumoniae isolated from pediatric infections in central Greece. Clinical Microbiology and Infection. 1998;4:695–700. doi: 10.1111/j.1469-0691.1998.tb00654.x. [DOI] [PubMed] [Google Scholar]
- 38.Syrogiannopoulos GA et al. Resistance patterns of Streptococcus pneumoniae from carriers attending day-care centers in southwestern Greece. Clinical Infectious Diseases. 1997;25:188–194. doi: 10.1086/514526. [DOI] [PubMed] [Google Scholar]
- 39.Sá-Leão R et al. Carriage of internationally spread clones of Streptococcus pneumoniae with unusual drug resistance patterns in children attending day care centers in Lisbon, Portugal. Journal of Infectious Diseases. 2000;182:1153–1160. doi: 10.1086/315813. [DOI] [PubMed] [Google Scholar]
- 40.Millar MR et al. Carriage of antibiotic-resistant bacteria by healthy children. Journal of Antimicrobial Chemotherapy. 2001;47:605–610. doi: 10.1093/jac/47.5.605. [DOI] [PubMed] [Google Scholar]
- 41.Porat N et al. Emergence of penicillin-nonsusceptible Streptococcus pneumoniae clones expressing serotypes not present in the antipneumococcal conjugate vaccine. Journal of Infectious Diseases. 2004;190:2154–2161. doi: 10.1086/425908. [DOI] [PubMed] [Google Scholar]