SUMMARY
Dispersed community outbreaks of Shigella sonnei have occurred cyclically among traditionally observant Jews in the United States. In February 2000, we investigated a S. sonnei outbreak in one Jewish community in New York City. To determine risk factors for introduction of infection into households, we conducted a cohort study of households to compare risk factors for illness among primary subjects within households and age-matched well siblings. Isolates were subtyped by pulsed-field gel electrophoresis (PFGE). We used a random effects model to assess extra-household vs. intra-household transmission in households with multiple ill household members. Daycare or pre-school attendance [matched odds ratio (mOR) 16·1, P<0·001] and age <60 months (mOR 6·3, P<0·001) were independently associated with index subject illness. Outbreak isolates were closely related by PFGE analysis to the strain previously observed in Jewish community outbreaks. The random effects model strongly indicated that multiple illnesses in a single household are due to secondary transmission. Disease containment efforts should focus on reducing Shigella transmission in childcare settings and within homes.
BACKGROUND
Shigella sonnei is a major cause of diarrhoeal illness in the United States, causing an estimated 450 000 cases annually [1]. Transmission is largely person-to-person, occurring commonly in infants and young children [1, 2]. Community outbreaks of shigellosis have been frequently associated with daycare and school attendance; common-source outbreaks are rare [3, 4]. Resistance to antimicrobial agents emerged in the United States in the 1980s and has been increasing. Of 367 isolates tested by CDC's National Antimicrobial Resistance Monitoring System during 1999–2000, 79% were resistant to ampicillin and 53% were resistant to trimethoprim–sulfamethoxazole (TMP–SMX), the historically recommended antibiotics for treatment of severe illness [5, 6].
Large, protracted, multi-state outbreaks of shigellosis in traditionally observant Jewish communities, affecting mostly young children, were identified in the United States during 1986–1987 and again during 1994–1996 [7, 8]. In 1986, a series of outbreaks in traditionally observant Jewish communities in six cities affected over 1900 persons and lasted 8 months. Investigators did not identify a common source or risk factor for infection, and transmission was presumed to be person-to-person. Over 25% of outbreak-associated isolates tested were resistant to ampicillin, and TMP–SMX resistance emerged during the course of the outbreak [7]. In 1994, a molecularly distinct strain of S. sonnei caused a single, protracted outbreak in traditionally observant Jewish communities in eight cities, affecting over 1000 persons and lasting 13 months [8]. Investigators did not identify a common source or risk factors for infection within communities. Most isolates tested were resistant to both ampicillin and TMP–SMX; these isolates were resistant to more antimicrobial agents than isolates obtained from contemporaneous sporadic cases of infection from outside the affected communities [8].
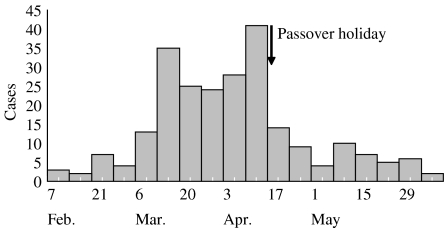
Between February and July 2000, the New York City Department of Health and Mental Hygiene (NYCDOHMH) identified 246 cases of culture-confirmed S. sonnei infection (Fig. 1) in one neighbourhood (population 60 000), home to a traditionally observant Jewish community, and most were children <5 years old. We conducted an epidemiological study designed to identify risk factors for introduction of S. sonnei into traditionally observant households, risk factors for transmission within households, the status of antimicrobial resistance and the role of antimicrobial therapy in illness treatment.
Fig. 1.
S. sonnei cases, by week of isolate collection, from one neighbourhood in New York City, 2000.
METHODS
Case ascertainment and hypothesis generation
Cases were identified from laboratory isolates with identifying information including an area zip code. In June 2000, we visited affected households and interviewed mothers of ill children, and toured local daycare centres and schools to identify potential risk factors for further evaluation.
Study design
Cohort study
To better understand the extent of and risk factors for intra-household Shigella transmission, we conducted a cohort study of affected households. For this study, a case household was defined as illness in a traditionally observant Jewish household in the affected neighbourhood, with at least one person aged <18 years ill with culture-confirmed S. sonnei infection, whose stool was obtained for culture between 1 February and 30 June 2000. An index subject was defined as the first case-household resident ill with culture-confirmed S. sonnei infection. A primary subject was defined as the first case-household resident ill with diarrhoeal illness (⩾3 loose stools in 24 h). A secondary case was defined as diarrhoea (⩾3 loose stools in 24 h) in a case-household resident with onset after the primary subject's illness. In November 2000, we contacted affected households by telephone, using a standard questionnaire. An adult household member identified the primary subject, numbers of ill persons in the home, illness onset dates, household characteristics, family demographics, and behaviours.
Case-control study
To test the hypothesis that daycare and preschool attendance was associated with the first illness in affected households, we conducted a nested case-control study comparing risk factors for illness among primary subjects and three well siblings nearest in age.
Recruitment
In September 2000, community leaders published a letter in the weekly local Yiddish newspaper, encouraging community participation, assuring anonymity, and promising feedback of study results.
Random effects model
To determine whether, in households with more than one case, the later cases were caused by secondary intra-household transmission, we created a random effects model. Random effects models are used by investigators to identify the variability of a response of a random sample of a population to multiple potential exposures within that population. In this model, we designated the subject having the earliest illness onset in the outbreak as the index case for the community. We compared the variance in lag times from community index subject's illness onset to the illness onsets of household primary subjects across households with the variance in lag times from each household primary subject's illness onsets to the illness onsets of subsequent cases within that household.
Laboratory assays
Stool samples were cultured for Shigella by standard methods. Antimicrobial susceptibility testing was performed on fresh overnight S. sonnei cultures using agar disc diffusion (Kirby–Bauer) methods according to standard procedures. Inhibition zone sizes were interpreted as sensitive, intermediate, or resistant according to current NCCLS guidelines [9].
For pulsed-field gel electrophoresis, bacterial DNA was extracted and immobilized in agarose plugs according to protocols standardized by PulseNet [10]. Genomic DNA in plugs was restricted with XbaI restriction enzyme (Promega, WI, USA) and electrophoresed overnight in a Chef DRII (Bio-Rad, Hercules, CA, USA) using the following parameters: 6 V/cm at 14°C for 18 h, with initial and final switch times of 2·2 s and 54·2 s respectively. Following electrophoresis, gels were stained in ethidium bromide (Sigma, St. Louis, MO, USA) and photographed under UV transillumination, and the resulting DNA fingerprints were compared.
Statistical analysis
Data were analysed with Epi-Info version 6.0 (CDC, Atlanta, GA, USA) and SAS version 8.0 (SAS Institute, Cary, NC, USA). Mantel–Haenszel matched odds ratios (mOR) and exact 95% confidence intervals for the maximum-likelihood estimate (MLE) of the odds ratio were calculated.
RESULTS
Case ascertainment and hypothesis generation
Of 246 culture-confirmed cases during the outbreak we were able to interview 96 (41%). Interviews with mothers in four affected households revealed that the first illness onset in a household occurred in a child attending daycare or pre-school, with subsequent illness in other household members. School administrators reported that >90% of children in the community began daycare by age 2 years. Of five facilities toured, four were private and religious, with limited toileting/handwashing facilities.
Cohort study
Descriptive epidemiology
Among the 96 case-patients for whom an interview was possible, median age was 42 months (range 8–160); 82% attended daycare or kindergarten. The median duration of illness was 3·6 days (range 1–9). Prominent symptoms included diarrhoea (94%), abdominal cramping (76%), fever (68%) and bloody diarrhoea (55%). Two persons were hospitalized; none died. Fifty-six (58%) ill persons received antimicrobial therapy. The number of daycare or school days missed by ill attendees who received antibiotics (median 5, range 1–15) was not statistically significant from those who did not receive antibiotics (median 5, range 1–14, P=0·54).
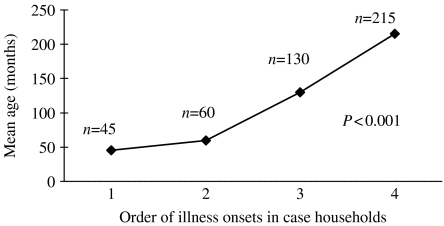
The first person ill in the household (household primary case) was also the household index case, that is, was the first laboratory-confirmed S. sonnei infection, in 52 (54%) households. For the remaining households, the primary case was not laboratory confirmed, but a subsequently ill household member did have confirmed S. sonnei infection. The median number of people living in the home was 6 (range 3–13). In 56 (58%) households, ⩾3 persons shared each bedroom. In 83 (87%) households, New York Medicaid was the primary insurer for children living in the home. Fifty-six case-households (58%) reported >1 person ill with diarrhoea during the outbreak. In these households, 22% of family members developed diarrohea. The median number of days between the illness onset of the primary subject and the next ill person was 5 days (range 2–15). Within case households, a means analysis of the age of ill persons, grouped by timing of illness onset, showed that the median age of primary subjects was 42 months, and that subsequently ill persons were significantly older (Fig. 2).
Fig. 2.
Means analysis of age of illness onset within households.
Household risk factors for intra-household infection transmission
Case-households with >1 ill person were compared with case-households having a single ill person with respect to the presence of younger children and the number of bathrooms in the home. By univariate and multivariate analysis, no statistically significant differences were found between households having >1 ill person and those having a single ill person.
Case-control study
Risk factors for household illness introduction
Among the 52 households in which the household index case had culture-confirmed S. sonnei infection, when risk factors for illness among index subjects were compared with those of three well siblings nearest in age, by univariate analysis, daycare and pre-school attendance and age <60 months were significantly associated with index subject illness (Table). Travel and attendance at social gatherings in the community or in the home were not associated with illness. By multivariate analysis, daycare or pre-school attendance and age <60 months were independently associated with index subject illness (Table).
Table.
Risk factors for illness among primary subject cases (n=52) and their three nearest-age siblings
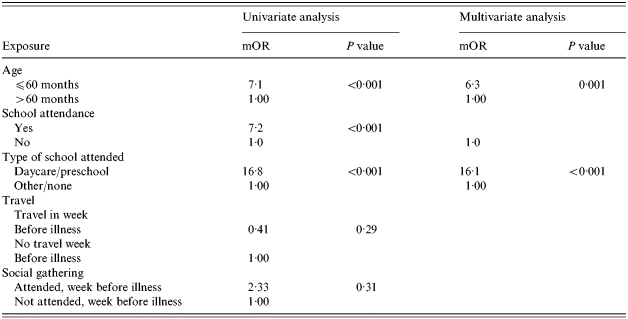
mOR, Matched odds ratio.
Random effects model
The ratio of the variance in lag times from the community index subject's illness onset to the illness onsets of household primary subjects across households, compared to the variance in lag times from the household primary subjects' illness onsets to the illness onsets of subsequent cases within households, was 26:1. This indicates a tight temporal clustering of primary subjects and subsequent illness onset dates within households, compared to primary subject illness onset dates among separate households.
Laboratory isolate testing
Pulsed-field gel electrophoresis (PFGE) subtyping
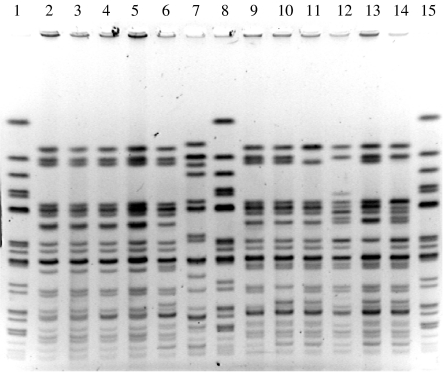
Of 246 cases reported in this outbreak, a convenience sample of 14 isolates was selected and subtyped by PFGE. All exhibited highly related PFGE patterns, most differing from each other by three or fewer bands. The PFGE patterns from isolates of this outbreak closely resembled those from isolates in previous outbreaks in a nearby traditionally observant Jewish community (Fig. 3) and from more recent outbreaks in non-Jewish communities [11].
Fig. 3.
Pulsed-field gel electrophoresis of Shigella sonnei digested by XbaI restriction enzyme. Lanes 1, 8, 15: standards. Lanes 2–5: isolates from outbreak described in this paper (2000). Lanes 6, 7, 9–14: outbreaks from a neighbouring traditionally observant Jewish community (1997).
Antimicrobial resistance
A convenience sample of 15 S. sonnei isolates associated with the outbreak were tested for antimicrobial susceptibility. All isolates were resistant to ampicillin and TMP–SMX, and two isolates also exhibited intermediate resistance to cefoperazone. All isolates were susceptible to fluoroquinolones, tetracycline, third-generation cephaplosporins and chloramphenicol.
DISCUSSION
This large community outbreak of shigellosis was caused by a strain of S. sonnei closely related to the strain that caused the outbreak in traditionally observant Jewish communities in eight cities in 1994–1996, and has caused illness in Jewish and non-Jewish communities since then [8]. Antimicrobial resistance in isolates from outbreaks in these communities remains high. We found that the main risk factor for introduction of S. sonnei infection into traditionally observant Jewish households in this outbreak was daycare and pre-school attendance. We also found that secondary transmission within households was common.
The association between primary subject illness and daycare and pre-school attendance is supported by the age of household primary subjects, the comparison of primary subject ages to the ages of persons with subsequent illness in the household, and the strong association between daycare and pre-school attendance and illness among index subjects compared with their well siblings nearest in age. The high variance ratio between illness onsets within affected households compared with illness onsets among separate affected households highlights the important role secondary transmission within households played in propagating illness.
Shigellosis is common in pre-school-aged children, and has been associated with several sustained epidemics in daycare and pre-school settings in the United States and Israel [3, 12]. The poorly developed personal hygiene skills of toddler children and the low infectious dose for S. sonnei transmission, often frustrate infection control measures in this population [13]. The decline in weekly numbers of new cases of infection as identified by Shigella laboratory isolates shortly after the Passover holiday week, during which children stayed home from school, suggests Shigella transmission disruption similar to that seen in other daycare centre outbreaks during school holidays, and suggests facility closure as a strategy for containment [7].
All subtyped S. sonnei isolates from this outbreak demonstrated closely related PFGE patterns. Slight variations in PFGE patterns of S. sonnei isolates are frequently observed in the course of epidemiologically defined outbreaks. The S. sonnei PFGE patterns identified in this outbreak closely resemble those that caused the 1994–1996 multistate outbreak among traditionally observant Jews [8]. The periodicity of Shigella outbreaks, every 5–6 years, may reflect a natural cycle of low-level endemic transmission erupting into outbreaks once a new cohort of young children without previous shigellosis infections enters preschool or school.
Antimicrobial resistance in tested isolates was high. As children remained home from daycare on average 2 days after diarrhoea resolved, it is difficult to assess the impact antibiotic therapy may have had on primary transmission in daycare centres. Routine use of antimicrobial therapy during shigellosis outbreaks remains controversial, and treatment of mild cases of illness in the face of increasing antimicrobial resistance remains of questionable value. New York City's traditionally observant Jewish community leaders were valuable partners in this study. They provided background information to the investigators and encouraged community participation.
Study findings were presented at a community meeting, which was attended by rabbis, paediatricians, school administrators and others, and where strategies for prevention and control of Shigella outbreaks were discussed
There were several limitations to this study. The delay of months between the outbreak period and household interviews may have resulted in recall bias. Approximately 59% of persons identified by laboratory surveillance were not able to be interviewed due to inability to determine telephone contact numbers, patient or parent refusal or unavailability during the survey period. These households may or may not have been representative of the overall affected group, potentially affecting strengths of associations. Furthermore, this study did not attempt to identify risk factors for infection within the daycare centres and pre-schools, and thus we could not draw conclusions about potential mechanisms for Shigella transmission in these institutions.
The results of this investigation suggest that the greatest burden of disease transmission in traditionally observant Jewish communities occurs in the daycare and pre-school setting, similar to the source of exposure in other communities. Secondary transmission within households significantly adds to the burden of illness. Intensive educational outreach regarding measures to control the transmission of diarrhoeal illness in household and daycare settings during shigellosis outbreaks in other communities has been shown to be effective in shortening sustained outbreaks [3]. In the absence of a vaccine, a culturally appropriate public health educational programme integrating proven strategies used in other Shigella outbreaks, such as educating parents and teachers about control measures including, directly observing handwashing with soap, aggressive decontamination of shared toys and other items, and cohorting of infected children, adapted to the unique features of the traditionally observant Jewish community may prove similarly effective in reducing shigellosis among young children in this population [3, 4, 14].
DECLARATION OF INTEREST
None.
REFERENCES
- 1.Gupta A et al. Laboratory-confirmed Shigellosis in the United States, 1989–2002: epidemiologic trends and patterns. Clinical Infectious Diseases. 2004;38:1372–1377. doi: 10.1086/386326. [DOI] [PubMed] [Google Scholar]
- 2.DuPont H, Mandell GL, Douglas R, Bennet J. Principles and Practices of Infectious Diseases. New York: Churchill Livingstone; 1990. Shigella species (bacillary dysentery) pp. 2033–2038. : pp. [Google Scholar]
- 3.Mohle-Boetani J et al. Community-wide shigellosis: control of an outbreak and risk factors in child day-care centers. American Journal of Public Health. 1995;85:812–816. doi: 10.2105/ajph.85.6.812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shane A et al. Sharing Shigella: risk factors for a multicommunity outbreak of shigellosis. Archives of Pediatric and Adolescent Medicine. 2003;157:602–603. doi: 10.1001/archpedi.157.6.601-b. [DOI] [PubMed] [Google Scholar]
- 5.CDC www.cdc.gov/narms/annual/2000/annual_pdf.com. www.cdc.gov/narms/annual/2000/annual_pdf.com . NARMS 2000 Annual Report. ( ). Accessed 21 July 2004.
- 6.Sivapalasingam S Increasing antimicrobial resistance among shigella isolates in the United States, 1999–2000. The 39th Annual Meeting of the Infectious Diseases Society of America; San Francisco, CA: 2001. [Google Scholar]
- 7.CDC. Multistate outbreak of Shigella sonnei gastroenteritis – United States. Morbidity and Mortality Weekly Report. 1987;36:440–442. 448–449. [PubMed] [Google Scholar]
- 8.Sobel J et al. A prolonged outbreak of Shigella sonnei infections in traditionally observant Jewish communities in North America caused by a molecularly distinct bacterial subtype. Journal of Infectious Diseases. 1998;177:1405–1408. doi: 10.1086/517825. [DOI] [PubMed] [Google Scholar]
- 9.NCCLS. Performance Standards for Antimicrobial Susceptibility Testing, Eleventh Informational Supplement. NCCLS; Pennsylvania: 2001. . NCCLS Document M100-S11. [Google Scholar]
- 10.Ribot EM et al. Rapid pulsed-field gel electrophoresis protocol for subtyping of Campylobacter jejuni. Journal of Clinincal Microbiology. 2001;39:1889–1894. doi: 10.1128/JCM.39.5.1889-1894.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.CDC. Day care-related outbreaks of rhamnose-negative Shigella sonnei – six states, June 2001–March 2003. Morbidity and Mortality Weekly Report. 2004;53:60–63. [PubMed] [Google Scholar]
- 12.Lerman Y et al. Epidemic spread of Shigella sonnei shigellosis and evidence for development of immunity among children attending day-care centers in a communal settlement (kibbutz) Journal of Clinical Microbiology. 1994;32:1092–1094. doi: 10.1128/jcm.32.4.1092-1094.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Herbert I et al. Inoculum size in shigellosis and implications for expected mode of transmission. Journal of Infectious Diseases. 1999;159:1126–1128. doi: 10.1093/infdis/159.6.1126. [DOI] [PubMed] [Google Scholar]
- 14.Tauxe R et al. Control of day care shigellosis: a trial of convalescent day care in isolation. American Journal of Public Health. 1986;76:627–630. doi: 10.2105/ajph.76.6.627. [DOI] [PMC free article] [PubMed] [Google Scholar]