SUMMARY
The antimicrobial resistance profiles of Campylobacter isolates recovered from a range of retail food samples (n=374) and humans (n=314) to eight antimicrobial compounds were investigated. High levels of resistance in food C. jejuni isolates were observed for ceftiofur (58%), ampicillin (25%) and nalidixic acid (17%) with lower levels observed for streptomycin (7·9%) and chloramphenicol (8·3%). A total of 80% of human C. jejuni isolates were resistant to ceftiofur, while 17% showed resistance to ampicillin and nalidixic acid, 8·6% to streptomycin and 4·1% to chloramphenicol. Resistance to clinically relevant antimicrobials such as erythromycin, ciprofloxacin and tetracycline was 6·7, 12, and 15% respectively for all food isolates and was similar to corresponding resistance prevalences observed for human isolates, where 6·4, 12 and 13% respectively were found to be resistant. Comparisons of C. jejuni isolates in each location showed a high degree of similarity although some regional variations did exist. Comparison of total C. jejuni and C. coli populations showed minor differences, with C. jejuni isolates more resistant to ampicillin and ceftiofur. Multidrug resistance patterns showed some profiles common to human and clinical isolates.
INTRODUCTION
Campylobacter infection has been well documented as being one of the most common causes of human gastroenteritis worldwide [1, 2]. Symptoms can vary from mild self-limiting enterocolitis lasting 24 h to more severe illness including diarrhoea, abdominal cramps and vomiting which can last up to 10 days. A small percentage of cases require medical intervention with erythromycin most commonly prescribed, however, in those immunosuppressed or with chronic intestinal disorders, Campylobacter infection can have more serious consequences and more long-term antibiotic therapy may be necessary [3, 4]. Sequelae although rare in healthy individuals, can include Guillain–Barré syndrome and Reiter syndrome [5, 6].
In the United States, an estimated 1·9–2·4 million cases of human campylobacteriosis occur each year [5, 7]. The incidence on the island of Ireland was reported at a crude incidence rate (CIR) of 39·9/100 000 in 2003 [8] in the Republic of Ireland and a CIR of 43·6/100 000 population in Northern Ireland [9]. The reported annual incidence rate of human Campylobacter infections in countries within the European Union in 1997 varied between 9·5 and 108 cases/100 000 [10].
Food animals especially poultry are universally recognized as the principal reservoirs for campylobacters, as the organism asymptomatically colonizes their gastrointestinal tracts. Foods of animal origin can subsequently become contaminated with these pathogens during the slaughter and processing stages [11]. Live poultry in particular have been identified as major reservoirs of Campylobacter jejuni [12]. Significant rates of intestinal carriage have also been reported in other food animal species including cattle (79%), pigs (100%) and sheep (92%) [13–15].
The use of antimicrobial agents in animals and humans has resulted in the emergence and dissemination of resistant bacteria [16]. The emergence of resistance to fluoroquinolones and macrolides which are important in human medicine, and particularly, the emergence of multi-antibiotic resistance in campylobacters has caused concern worldwide [17]. The acceleration in the prevalence of multidrug resistant bacteria in food animals, food of animal origin and humans has overtaken new drug development and gives the prospect of untreatable infections [18–20]. Surveillance of resistance trends in defined bacterial populations provides valuable data on the links between antimicrobial use and emerging antimicrobial public health problems. To this end our study aims to examine the resistance patterns of Campylobacter isolates of food origin obtained at retail level and human isolates obtained from clinical cases over a defined time period in three major population centres on the island of Ireland.
MATERIALS AND METHODS
Food isolate collection
Retail food samples were collected on a monthly basis over a 20-month period between March 2001 and October 2002. The samples were purchased from a range of supermarkets and butchers shops in three population centres, namely, Dublin, Galway and Belfast. Typically, 20–30 samples from each city were purchased every month and forwarded to the Veterinary Public Health and Food Safety Laboratory, School of Agriculture, Food Science and Veterinary Medicine, UCD. Isolates were recovered from a range of food types analysed during this period including chicken, duck, turkey, lamb, pork, beef, seafood (oysters), mushrooms, and chicken liver paté. All samples were transported to the Dublin laboratory on ice, and processed in the laboratory within 24 h of purchase.
Sample preparation
For all solid foods, including meat and poultry, 10 g from each sample was aseptically removed using sterile scissors and forceps. Samples were placed in 90-ml volumes of Preston broth (Mast Diagnostics, Bootle, Merseyside, UK and Oxoid, Basingstoke, Hampshire, UK) in sterile plastic bags and processed for 1 min in a stomacher (Lab Blender 400, Seward Medical, Thetford, Norfolk, UK). Both the stomached samples and broths were then placed in sterile plastic disposable 100-ml universal containers and additional broth added as required in order to minimize head space between the liquid and the container lids. Liquid food samples, including raw milk and yoghurts were selectively enriched by adding 50-ml volumes of sample to sterile sample bottles containing an equal volume of double-strength Preston broth.
Microbiological analysis and identification
Preston broths were prepared in accordance with the formulation developed by Bolton et al. [21] and included growth and antimicrobial selective supplements, as well as 5% (v/v) lysed horse blood. Following initial processing in a stomacher, all samples were selectively enriched in the Preston broths for 48 h at 42±1°C. All enriched samples were subsequently subcultured on to selective solid media, modified charcoal cefoperazone deoxycholate agar (mCCDA; Mast Diagnostics, and Oxoid). The mCCDA plates were incubated for 48 h at 42±1°C under a microaerophilic atmosphere, which was achieved using gas jars and catalyst-free gas packs (bioMérieux, Marcy l'Etoile, France). Suspect colonies on solid media were subcultured on to Columbia blood agar containing 5% (v/v) horse blood, which were again incubated for 48 h at 42±1°C in a microaerophilic atmosphere. Colonies were examined morphologically and Gram stained as presumptive identification of positives. Final confirmation and speciation was carried out using the CampID biochemical profiling system (Mast Diagnostics) or a multiplex PCR assay as previously described [22].
Clinical isolate collection
Human clinical Campylobacter isolates were either collected or delivered from the collaborating Public Health Laboratories in Dublin, Belfast and Galway to the Veterinary Public Health and Food Safety Laboratory in UCD during this period. Isolates were transported to the laboratory on Amies medium transportation swabs (Copan Innovation, Brescia, Italy) or on Protect beads (TSC, Heywood, Lancashire, UK). They were subcultured onto Columbia blood agar containing 5% (v/v) horse blood, and incubated for 48 h at 42±1°C in a microaerophilic atmosphere. Isolates were identified using gross colony morphology and subsequently speciated using biochemical profiling.
Phenotypic antimicrobial resistance profiling
The disc diffusion method as recommended in the National Committee for Clinical Laboratory Standards (NCCLS) guidelines [23] was chosen to investigate the antimicrobial resistance patterns of 314 human and 374 food isolates. The disc diffusion method was a reliable and inexpensive method for monitoring the prevalence of the large number of Campylobacter strains used in this study, as it compares well to other resistance methods: E-testing, Microdilution broth method or Agar dilution methods [24–26]. Sensitivity determinations by disc diffusion were made in accordance with the guidelines recommended by NCCLS with the commonly used standards E. coli ATCC 25922, S. aureus ATCC 29213 and Pseudomonas aeruginosa 2783 included.
Campylobacter food and clinical isolates were grown on Columbia agar (Oxoid) containing 5% (v/v) lysed horse blood incubated microaerophilically at 42°C for 48 h. Cultures were prepared from a fresh (non-frozen), pure 48 h culture diluted with sterile distilled water, to give an inoculum with an equivalent cell density to a 0·5 McFarland turbidity standard, and then swabbed evenly onto agar plates and allowed to dry. The following discs (concentrations in parentheses) were then applied to each agar plate: ampicillin (10 μg), ciprofloxacin (5 μg), chloramphenicol (10 μg), erythromycin (10 μg), streptomycin (25 μg), tetracycline (30 μg), and for some isolates (n=426) ceftiofur (30 μg) (Oxoid). The breakpoints were measured by calipers and were interpreted according to those recommended by NCCLS [23].
Statistical analysis
The prevalence of antimicrobial resistance in Campylobacter isolates was compared statistically by geographical location using χ2 analysis. Statistical significance was defined at the P⩽0·05 level. All statistical analysis was carried out using statview version 5.0.1 (SAS Institute, Cary, NC, USA).
RESULTS
Campylobacter speciation
A total of 314 clinical isolates were collected from the collaborating Public Health Laboratories during the period 2001–2002. Campylobacter jejuni accounted for 92·3% (n=290) of isolates with Campylobacter coli accounting for the remaining 7·6% (n=24) of clinical isolates. From available clinical data of the Belfast isolates, approximately 63% were acquired from patients presenting to their general practitioner in the community, with the remainder (37%) being recovered from hospital in-patients, approximately 59%, of patients were female and 41% were male. The reported rate of hospitalization for Dublin patients, where information was available, was 57·3%, while 56·4% of patients were female and 43·6% were male.
Of the 374 food isolates assayed 67·7% (n=253) were C. jejuni, and 32·3% (n=121) were C. coli.
Phenotypic antimicrobial resistance profiles of clinical isolates
The prevelance of antimicrobial resistance among human clinical Campylobacter isolates collected during 2001–2003 in Dublin, Belfast and Galway are presented in Table 1. High levels of resistance were observed among isolates to several of the antimicrobials tested. A total of 17% of C. jejuni isolates were resistant to ampicillin, 13% to ciprofloxacin, 17% to nalidixic acid, 10% to tetracycline and 80% were resistant to ceftiofur. Lower frequencies of resistance were recorded amongst these isolates to erythromycin (6·2%), streptomycin (8·6%) and chloramphenicol (4·1%). C. coli isolates showed high resistance to ceftiofur (82%), ampicillin (17%), tetracycline (12·5%) and nalidixic acid (12·5%). The C. coli isolates showed a lower resistance to both ciprofloxacin (8·3%) and to chloramphenicol (4·2%).
Table 1.
Antimicrobial resistance of human Campylobacter isolates collected between 2001 and 2002 in Dublin, Belfast and Galway
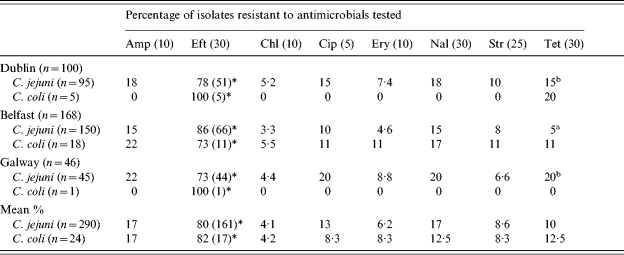
Amp, Ampicillin; Eft, ceftiofur; Chl, chloramphenicol; Cip, ciprofloxacin; Ery, erythromycin; Nal, nalidixic acid; Str, streptomycin; Tet, tetracycline (μg/disc).
Figures in parentheses show the number of isolates tested for resistance to Eft (ceftiofur).
Superscripts a, b denote statistical significance between values for an antimicrobial for C. jejuni isolates (P⩽0·05) (χ2 analysis).
Phenotypic antimicrobial resistance profiles of food isolates
The prevalence of antimicrobial resistance of Campylobacter isolates recovered from foods sampled in 2001 and 2002 is summarized in Table 2. In total, 374 isolates of food origin were subjected to antimicrobial resistance screening using a range of antimicrobials, including clinically relevant chemotherapeutics. In general, a large number of isolates were resistant to a range of the antimicrobials tested. For example a total of 58% of C. jejuni isolates tested were resistant to ceftiofur and high levels of resistance were also observed for ampicillin (25%), ciprofloxacin (17%), nalidixic acid (17%) and tetracycline (12%). Lower prevalences of resistant isolates were observed when screened using erythromycin (6·7%), streptomycin (7·9%) and chloramphenicol (8·3%). In C. coli isolates a similar pattern of resistance was observed with ceftiofur (71%), ampicillin (27%), tetracycline (11%) and streptomycin (11%) giving the highest prevalences. Lower levels of resistance were found for ciprofloxacin (9%), nalidixic acid (8·3%), chloramphenicol (7·4%) and erythromycin (6·6%). The resistance profiles of C. jejuni and C. coli isolates from poultry and other foods are presented in Table 3. Most campylobacters of food origin isolated in the current study were obtained from raw poultry (chicken, turkey and duck) with other isolates obtained from beef, lamb and pork. A small number of isolates were also obtained from mushrooms (n=3), oysters (n=1) and chicken liver paté (n=1). Frequent resistance to the range of antimicrobials tested was observed among these isolates from non-poultry sources.
Table 2.
Antimicrobial resistance of Campylobacter isolates of food origin collected during 2001–2002 in Dublin, Belfast and Galway
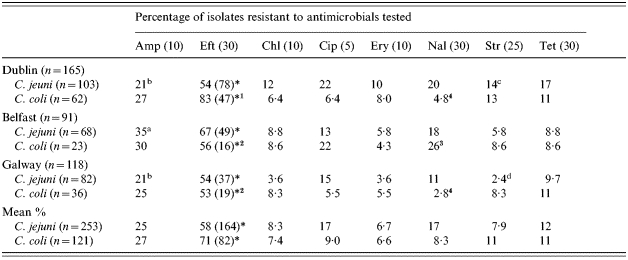
Amp, Ampicillin; Eft, ceftiofur; Chl, chloramphenicol; Cip, ciprofloxacin; Ery, erythromycin; Nal, nalidixic acid; Str, streptomycin; Tet, tetracycline (μg/disc).
Figures in parentheses show the number of isolates tested for resistance to Eft (ceftiofur).
Superscripts a, b, c, d denote statistical significance between values for an antimicrobial for C. jejuni isolates (P⩽0·05) (χ2 analysis). Superscripts 1, 2, 3, 4 denotes statistical significance between values of an antimicrobial for C. coli isolates (P⩽0·05) (χ2 analysis).
Table 3.
Antimicrobial resistance of Campylobacter isolates recovered from different food categories during 2001–2002 in Dublin, Belfast and Galway

Amp, Ampicillin; Eft, ceftiofur; Chl, chloramphenicol; Cip, ciprofloxacin; Ery, erythromycin; Nal, nalidixic acid; Str, streptomycin; Tet, tetracycline (μg/disc).
Figures in parentheses show the number of isolates tested for resistance to Eft (ceftiofur).
Includes beef, lamb, pork, shellfish, mushrooms and chicken liver paté.
Multidrug resistance
A considerable number of isolates were found to be resistant to more than one antimicrobial. For clinical isolates the percentage of C. jejuni isolates (n=290) resistant to 2, 3, 4, 5, 6, 7 and all 8 of the antimicrobioals tested was 11, 8, 4, 1, 0·34 and 0·34% respectively. Thus 24·7% were resistant to two or more antimicrobials. For C. coli isolates (n=24) multidrug resistance was observed to 2 (4·2%), 3 (8·3%), 5 (4·2%) and 6 (4·2%) antimicrobials. In food C. jejuni isolates (n=253) resistance to 2, 3, 4, 5, 7, and 8 antimicrobials was 16, 7·1, 3·2, 2·8, 2·4 and 0·79% respectively giving a total of 31·5% resistant to two or more antimicrobials. C. coli isolates (n=121) were resistant to 2 (16%), 3 (9%), 4 (1·6%), 5 (1·6%) and 7 (2·5%) antimicrobials. The most prevalent multidrug resistance profiles observed for C. jejuni isolates are shown in Table 4. Due to the high level of resistance shown by all C. jejuni isolates to ceftiofur this antimicrobial was not included when determining the data for Table 4.
Table 4.
Summary of multidrug resistance profiles for Campylobacter jejuni isolates of food and human origin during 2001–2002 in Dublin, Belfast and Galway
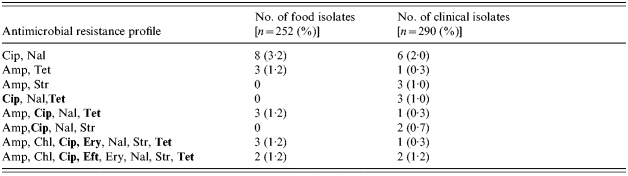
Clinically important antimicrobials indicated in bold type.
Cip, ciprofloxacin; Nal, nalidixic acid; Amp, Ampicillin; Tet, tetracycline; Str, streptomycin; Chl, chloramphenicol; Ery, erythromycin; Eft, ceftiofur.
When multidrug resistance to the therapeutically relevant antimicrobials erythromycin, ciprofloxacin and tetracycline were examined, we found 4·0% of food isolates and 2·2% of human isolates resistant to all three of these antibiotics and an additional 3·5 and 3·7% resistant to two of three antimicrobials. We found 4·0% of food isolates and 3·5% of clinical isolates were resistant to both erythromycin and ciprofloxacin.
Geographical variation
Although resistance patterns of clinical and food isolates were similar some variations were observed when geographical locations were compared. For example, tetracycline resistance in Belfast clinical C. jejuni isolates (5%) was significantly lower (P⩽0·05) than those originating from either Dublin (15%) or Galway (20%). Among food C. jejuni isolates resistance to ampicillin was significantly higher in Belfast (35%) than either Dublin (21%) or Galway (21%) (P⩽0·05). Streptomycin resistance in isolates obtained in Galway (2·4%) was significantly lower than those originating from Dublin (14%) (P⩽0·05). C. coli food isolates from Dublin showed significantly greater resistance to ceftiofur (83%) than either Galway (53%) or Belfast (56%) isolates (P⩽0·05). Resistance among Belfast isolates to nalidixic acid (26%) was significantly higher to either Dublin (4·8%) or Galway (2·8%) isolates (P⩽0·05).
When the total food C. jejuni (n=253) and C. coli populations (n=121) were compared it was found that C. jejuni isolates displayed significantly higher resistance to ciprofloxacin (17%, 9%) and nalidixic acid (17%, 8·3%) (P⩽0·05).
When C. jejuni food and clinical isolates were compared for each location there were no significant differences among Galway isolates. Dublin food and clinical C. jejuni isolates displayed significantly different susceptibilies to ceftiofur with 54 and 78% of isolates respectively found to be resistant (P⩽0·05). Equivalent isolates from Belfast showed significantly different resistance prevalences for ampicillin and ceftiofur (P⩽0·05) (Tables 1 and 2).
DISCUSSION
Overall similar resistance prevalences were observed to the range of antimicrobials used in the present study for both the 374 food and 314 human isolates, which suggests that emerging trends in Campylobacter resistance in foodstuffs is reflected in human isolates and is therefore of importance to human health. High prevalences of resistance in the isolates we studied were found for ceftiofur, ampicillin, tetracycline, nalidixic acid and ciprofloxacin among both groups of isolates. Lower levels of resistance were observed for erythromycin, streptomycin and chloramphenicol. This is similar to the trends found by Wilson [27]. Global surveillance of resistance among campylobacters of food origin using disc diffusion have shown widely varying profiles with ranges of 0–45% for erythromycin, 1–76% for tetracycline and 2–75% for ciprofloxacin reported [2, 28–30]. A similar variation occurs for human isolates with erythromycin resistance in the range of 0–24%, tetracycline 1–63% and ciprofloxacin 6–76%. [2, 25, 28, 31]. The observed variability in resistance reported in these studies is possibly due to differences in sample origin, laboratory methods applied and different antibiotic use patterns in human and veterinary medicine in the countries surveyed.
In the most recent studies on the island of Ireland, the reported resistance prevalences (by disc diffusion) of clinically relevant therapeutics in campylobacters of food origin have varied, ranging from 0 to 11% for erythromycin, 11 to 24% for tetracycline and 2·7 to 9% for ciprofloxacin [27, 32–36] while in our study we found 6·7, 12, and 15% respectively for all of the food camplyobacters studied. In contrast to the previously reported studies, our samples were obtained at retail level which possibly provides a more realistic assessment of the actual risk closer to the point of consumption compared to samples taken earlier in the food chain.
Samples were surveyed from three different densely populated regions of the island of Ireland. Although a high degree of similarity was evident some variations in the prevalence of resistance of campylobacters were observed within these populations when geographical locations were compared. For example, in food C. jejuni populations significantly higher resistance to ampicillin was observed in isolates originating from Belfast (35%) than in either Dublin (21%) or Galway (21%). For C. coli populations resistance to nalidixic acid was significantly higher in isolates obtained in Belfast (26%) than those from either Dublin (4·8%) or Galway (2·8%) (P⩽0·05). Resistance among Campylobacter isolates of human origin on the island of Ireland to clinically relevant antibiotics have been reported at rates ranging from 2 to 4·2% for erythromycin, 4 to 31% for tetracycline and 7 to 34% for ciprofloxacin [27, 32, 33, 35] this compares to 6·4%, 12% and 13% for the total clinical Campylobacter population we studied. While the resistance prevalence of isolates from the current study was greater for erythromycin and lower than most of the previously published data for ciprofloxacin and tetracycline this may be the result of the wider geographical and temporal spread of human isolates collected during the current investigation. Within our C. jejuni human isolate population, tetracycline resistance was significantly higher for Dublin (15%) and Galway (20%) strains than in corresponding clinical isolates originating from Belfast (5%) (P⩽0·05) perhaps reflecting differences in regional antimicrobial prescribing patterns.
When we compared the resistance of C. jejuni food and clinical isolates in each location there was a high degree of similarity although some regional variations did exist. Dublin C. jejuni isolates differed in resistance to ceftiofur with clinical isolates (78%) showing significantly greater resistance than food isolates (54%). Belfast food C. jejuni isolates were significantly higher than corresponding clinical isolates for ampicillin with prevalences of 35 and 15% observed respectively (P⩽0·05). Clinical isolates (86%) were found to be more resistant to ceftiofur than corresponding food isolates (67%) (P⩽0·05). Overall our results show that the prevalence of resistance in food and clinical isolates to the antimicrobials tested were similar which suggests that surveillance of antimicrobial resistance in food populations would provide an accurate indication of resistance in corresponding human clinical campylobacters. This approach may be useful as a broad subtyping method when used in conjunction with a suitable molecular typing technique.
This hypothesis was supported by examination of the multi-resistant profiles of C. jejuni food and human isolates. A number of common multi-resistant profiles were observed between food and clinical C. jejuni populations. For example both groups contained isolates resistant to Cip Nal; Amp Tet and Amp Cip Nal Tet combinations (Table 4). A number of food and clinical isolates also showed resistance to seven and eight of the antimicrobials tested including combinations containing the clinically relevant antimicrobials (Cip, Ery, Tet). We found 4·0% of food isolates and 2·2% of human isolates resistant to all three of these antimicrobioals and 4·0% of food isolates and 3·5% of clinical isolates resistant to both erythromycin and ciprofloxacin. This is an important observation as although most clinical cases of campylobacteriosis are self-limiting, antimicrobial therapy may be needed to treat more severe or recurrent infections. In these instances erythromycin is frequently the preferred antimicrobial, with fluoroquinolones and tetracyclines also used to a lesser extent. Concern regarding the loss of therapeutic effectiveness has been expressed in recent studies that have shown that the mean duration of illness can be longer in patients infected with quinolone resistant campylobacters, than in those infected with quinolone-susceptible strains [37, 38], which may be indicative of increased virulence in these resistant strains [20].
A wide variation in resistance profiles was observed for food isolates originating from different food animals. Overall, Campylobacter isolates from both poultry and pork samples demonstrated a higher and broader spectrum of resistance to the antimicrobials tested in this study than those from beef or lamb. This could be a result of differences in production systems and husbandry practices applied to produce these food animals [39]. The intensive high throughput systems used to produce both pigs and poultry may result in a greater need to mass medicate animals to treat infectious diseases thereby exerting increased selective pressures on enteric pathogens, including Campylobacter [40]. Moreover, the high stocking densities associated with these production systems may facilitate the dissemination of these resistant organisms within and between flocks/herds, a view which is supported by Turnidge [41].
On the island of Ireland beef and lamb production is primarily extensive and grass based. Animals are treated with antimicrobials on an individual basis as required. Intensive production systems are primarily used to rear pigs and poultry and mass medication is more likey to be used. Use of antimicrobials follows strict national and EU legislation and antimicrobials are used solely for therapeutic purposes with the exception of a small number of approved coccidostats used prophylatically in the poultry industry. No current data is available for antimicrobials' usage patterns in food animals or indeed in human medicine on the island of Ireland. The European Federation of Animal Health reported in 1998 that approximately 50% of all antibacterial agents used annually in the EU are given to animals [42]. However, Ungemach reported that antimicrobial administration to animals in Ireland was below the average usage for Europe [43]. Extensive antimicrobial residue monitoring is officially performed by the national regulatory agencies and non-compliance is extremely low (<1%) in Irish food animals [44] yet our study demonstrates the relatively high prevalence of resistance in campylobacters of food origin.
The World Health Organisation in their Global Strategy for Containment of Antimicrobial Resistance [18] advocates the strengthening of health and surveillance systems among other measures to evaluate and reduce risks. Such surveillance has led to restrictions in the use of certain antimicrobials agents in veterinary medicine including the banning of growth promoters like avoparcin by the EU and the recent ban on the use of enrofloxacin in poultry in the United States. The success of such control interventions has been demonstrated by significant reduction in the prevalence of vancomycin-resistant entercocci since the banning of avoparcin was enforced [20, 40].
The current study helps expand the baseline knowledge of resistance in local Campylobacter populations on the island of Ireland and to monitor the emerging resistance trends in isolates from foods and how they may be linked to human Campylobacter resistance patterns, particularly in relation to the clinically relevant antimicrobials and to emerging multidrug resistance. We conclude that ongoing surveillance of defined Campylobacter populations on the island of Ireland of human and food origin should be undertaken to monitor the progress of antibiotic resistance and to quantify the critical inhibitory levels in multidrug-resistant isolates in the interest of public health protection.
ACKNOWLEDGEMENTS
The authors gratefully acknowledge safeFood, the Food Safety Promotion Board (island of Ireland) who funded this research programme. Dr J. E. Moore is partly supported by a grant from the NI HPSS Research and Development Office (RRG 9.9).
DECLARATION OF INTEREST
None.
REFERENCES
- 1.Allos BM. Campylobacter jejuni infections: update on emerging issues and trends. Clinical Infectious Diseases. 2001;32:1201–1206. doi: 10.1086/319760. [DOI] [PubMed] [Google Scholar]
- 2.Ronner AC et al. Species identification by genotyping and determination of antibiotic resistance in Campylobacter jejuni and Campylobacter coli from humans and chickens in Sweden. International Journal of Food Microbiology. 2004;96:173–179. doi: 10.1016/j.ijfoodmicro.2004.03.017. [DOI] [PubMed] [Google Scholar]
- 3.Bodhidatta L et al. Bacterial enteric pathogens in children with acute dysentery in Thailand: increasing importance of quinolone-resistant Campylobacter. Southeast Asian Journal of Tropical Medicine and Public Health. 2002;33:752–757. [PubMed] [Google Scholar]
- 4.Boyanova L et al. Campylobacter infection in 682 Bulgarian patients with acute enterocolitis, inflammatory bowel disease, and other chronic intestinal diseases. Diagnostic Microbiology and Infectious Disease. 2004;49:71–74. doi: 10.1016/j.diagmicrobio.2003.12.004. [DOI] [PubMed] [Google Scholar]
- 5.Altekruse SF et al. Campylobacter jejuni – an emerging foodborne pathogen. Emerging Infectious Diseases. 1999;5:28–35. doi: 10.3201/eid0501.990104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bersudsky M et al. Lipopolysaccharides of a Campylobacter coli isolate from a patient with Guillain–Barre syndrome display ganglioside mimicry. Neuromuscular Disorders. 2000;10:182–186. doi: 10.1016/s0960-8966(99)00106-6. [DOI] [PubMed] [Google Scholar]
- 7.Tauxe RV. Emerging foodborne pathogens. International Journal of Food Microbiology. 2002;78:31–41. doi: 10.1016/s0168-1605(02)00232-5. [DOI] [PubMed] [Google Scholar]
- 8.Foley B, McKeown P. Camplyobacterosis on Ireland 2003. Epi-insight. 2005;6:2. [Google Scholar]
- 9.Communicable Disease Surveillance Centre, Northern Ireland . Laboratory reports of Campylobacter spp. (all specimen types). Northern Ireland: Communicable Disease Surveillance Centre, 2004 [Google Scholar]
- 10.European Commission Berlin, Germany: 1999. . Trends and sources of zoonotic agents in animals, feedstuffs, food and man in the European Union in 1997. Part 1. Document No. VI/8495/98-Rev.2 of the European Commission. : Community Reference Laboratory on the Epidemiolgy of Zoonoses, BgVV, [Google Scholar]
- 11.Anon 1999. . Antibiotic resistance in the European Union associated with therapeutic use of veterinary medicines. Report and Qualitative Risk Assessment by the Committee for Veterinary Medicinal Products. EMEA European Agency for the Evaluation of Medicinal Products,
- 12.Refregier-Petton J et al. Risk factors for Campylobacter spp. contamination in French broiler-chicken flocks at the end of the rearing period. Preventive Veterinary Medicine. 2001;50:89–100. doi: 10.1016/s0167-5877(01)00220-3. [DOI] [PubMed] [Google Scholar]
- 13.Nesbakken T et al. Occurrence of Yersinia enterocolitica and Campylobacter spp. in slaughter pigs and consequences for meat inspection, slaughtering, and dressing procedures. International Journal of Food Microbiology. 2003;80:231–240. doi: 10.1016/s0168-1605(02)00165-4. [DOI] [PubMed] [Google Scholar]
- 14.Stanley KN et al. Seasonal variation of thermophilic campylobacters in lambs at slaughter. Journal of Applied Microbiology. 1998;84:1111–1116. doi: 10.1046/j.1365-2672.1998.00450.x. [DOI] [PubMed] [Google Scholar]
- 15.Atabay HI, Corry JE. The isolation and prevalence of campylobacters from dairy cattle using a variety of methods. Journal of Applied Microbiology. 1998;84:733–740. doi: 10.1046/j.1365-2672.1998.00402.x. [DOI] [PubMed] [Google Scholar]
- 16.Anderson AD et al. Public health consequences of use of antimicrobial agents in food animals in the United States. Microbial Drug Resistance. 2003;9:373–379. doi: 10.1089/107662903322762815. [DOI] [PubMed] [Google Scholar]
- 17.Aarestrup FM, Engberg J. Antimicrobial resistance of thermophilic Campylobacter. Veterinary Research. 2001;32:311–321. doi: 10.1051/vetres:2001127. [DOI] [PubMed] [Google Scholar]
- 18.WHO Global Strategy for Containment of Antmicrobial Resistance. Geneva, Switzerland: Department for Communicable Disease Surveillance and Response, World Health Organization; 2001. [Google Scholar]
- 19.Lipsitch M, Singer RS, Levin BR. Antibiotics in agriculture: when is it time to close the barn door? Proceedings of the National Academy of Sciences USA. 2002;99:5752–5754. doi: 10.1073/pnas.092142499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shea KM. Antibiotic resistance: what is the impact of agricultural uses of antibiotics on children's health? Pediatrics. 2003;112:253–258. [PubMed] [Google Scholar]
- 21.Bolton FJ, Robertson L. A selective medium for isolating Campylobacter jejuni/coli. Journal of Clinical Pathology. 1982;35:462–467. doi: 10.1136/jcp.35.4.462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang G et al. Colony multiplex PCR assay for identification and differentiation of Campylobacter jejuni, C. coli, C. lari, C. upsaliensis, and C. fetus subsp. fetus. Journal of Clinical Microbiology. 2002;40:4744–4747. doi: 10.1128/JCM.40.12.4744-4747.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.NCCLS. Performance Standards for Antimicrobial Susceptibility Testing: Twelfth Information Supplement. Wayne, PA, USA: National Committee for Clinical Laboratory Standards; 2002. [Google Scholar]
- 24.Frediani-Wolf V, Stephan R. Resistance patterns of Campylobacter spp. strains isolated from poultry carcasses in a big Swiss poultry slaughterhouse. International Journal of Food Microbiology. 2003;89:233–240. doi: 10.1016/s0168-1605(03)00152-1. [DOI] [PubMed] [Google Scholar]
- 25.Alfredson DA, Akhurst RJ, Korolik V. Antimicrobial resistance and genomic screening of clinical isolates of thermophilic Campylobacter spp. from south-east Queensland, Australia. Journal of Applied Microbiology. 2003;94:495–500. doi: 10.1046/j.1365-2672.2003.01856.x. [DOI] [PubMed] [Google Scholar]
- 26.Gaudreau C, Gilbert H. Comparison of disc diffusion and agar dilution methods for antibiotic susceptibility testing of Campylobacter jejuni subsp. jejuni and Campylobacter coli. Journal of Antimicrobial Chemotherapy. 1997;39:707–712. doi: 10.1093/jac/39.6.707. [DOI] [PubMed] [Google Scholar]
- 27.Wilson IG. Antibiotic resistance of Campylobacter in raw retail chickens and imported chicken portions. Epidemiology and Infection. 2003;131:1181–1186. doi: 10.1017/s0950268803001298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pezzotti G et al. Occurrence and resistance to antibiotics of Campylobacter jejuni and Campylobacter coli in animals and meat in northeastern Italy. International Journal of Food Microbiology. 2003;82:281–287. doi: 10.1016/s0168-1605(02)00314-8. [DOI] [PubMed] [Google Scholar]
- 29.Ledergerber U et al. Risk factors for antibiotic resistance in Campylobacter spp. isolated from raw poultry meat in Switzerland. BMC Public Health. 2003;3:39. doi: 10.1186/1471-2458-3-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Uzunovic-Kamberovic S. Antibiotic susceptibility of Campylobacter jejuni and Campylobacter coli human isolates from Bosnia and Herzegovina. Journal of Antimicrobial Chemotherapy. 2003;51:1049–1051. doi: 10.1093/jac/dkg142. [DOI] [PubMed] [Google Scholar]
- 31.Gaudreau C, Gilbert H. Antimicrobial resistance of Campylobacter jejuni subsp. jejuni strains isolated from humans in 1998 to 2001 in Montreal, Canada. Antimicrobial Agents and Chemotherapy. 2003;47:2027–2029. doi: 10.1128/AAC.47.6.2027-2029.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Moore JE et al. Antibiotic resistance in Campylobacter spp. isolated from human faeces (1980–2000) and foods (1997–2000) in Northern Ireland: an update. Journal of Antimicrobial Chemotherapy. 2001;48:455–457. doi: 10.1093/jac/48.3.455. [DOI] [PubMed] [Google Scholar]
- 33.Lucey B et al. Trends in antimicrobial susceptibility among isolates of Campylobacter species in Ireland and the emergence of resistance to ciprofloxacin. Veterinary Record. 2002;151:317–320. doi: 10.1136/vr.151.11.317. [DOI] [PubMed] [Google Scholar]
- 34.Fallon R et al. Antimicrobial resistance of Campylobacter jejuni and Campylobacter coli isolates from broiler chickens isolated at an Irish poultry processing plant. Letters in Applied Microbiology. 2003;36:277–281. doi: 10.1046/j.1472-765x.2003.01308.x. [DOI] [PubMed] [Google Scholar]
- 35.Moore JE et al. Characterisation of fluoroquinolone-resistant Campylobacter species isolated from human beings and chickens. Veterinary Record. 2002;150:518–520. doi: 10.1136/vr.150.16.518. [DOI] [PubMed] [Google Scholar]
- 36.Oza AN et al. Antimicrobial susceptibility of Campylobacter spp. isolated from broiler chickens in Northern Ireland. Journal of Antimicrobial Chemotherapy. 2003;52:220–223. doi: 10.1093/jac/dkg333. [DOI] [PubMed] [Google Scholar]
- 37.Nelson JM et al. Prolonged diarrhea due to ciprofloxacin-resistant Campylobacter infection. Journal of Infectious Diseases. 2004;190:1150–1157. doi: 10.1086/423282. [DOI] [PubMed] [Google Scholar]
- 38.Engberg J et al. Quinolone-resistant Campylobacter infections: risk factors and clinical consequences. Emerging Infectious Diseases. 2004;10:1056–1063. doi: 10.3201/eid1006.030669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pepin M, Russo P, Pardon P. Public health hazards from small ruminant meat products in Europe. Revue Scientifique et Technique. 1997;16:415–425. doi: 10.20506/rst.16.2.1040. [DOI] [PubMed] [Google Scholar]
- 40.van den Bogaard AE, Stobberingh EE. Epidemiology of resistance to antibiotics. Links between animals and humans. International Journal of Antimicrobial Agents. 2000;14:327–335. doi: 10.1016/s0924-8579(00)00145-x. [DOI] [PubMed] [Google Scholar]
- 41.Turnidge J. Antibiotic use in animals – prejudices, perceptions and realities. Journal of Antimicrobial Chemotherapy. 2004;53:26–27. doi: 10.1093/jac/dkg493. [DOI] [PubMed] [Google Scholar]
- 42.FEDESA 1998. . European Federation of Animal Health. Press release on the European Union Conference: the microbial threat. Copenhagen,
- 43.Ungemach FR. Figures on quantities of antibacterials used for different purposes in EU countries and interpretation. Acta Veterinaria Scandinavica. 2000;41:89–89. [PubMed] [Google Scholar]
- 44.Anon Dublin, Ireland: 2005. . Press release no. 117/05. : Department of Agriculture and Food, [Google Scholar]


