SUMMARY
In April 2004, increased numbers of hepatitis A were noted in six neighbouring districts in Germany. Exploratory interviews showed that patients had consumed bakery products from company X where two employees had been diagnosed with hepatitis A in February. A case-control study of consumption of products of company X was carried out through telephone interviews. Altogether, 64 cases were identified. Fifty-two cases and 112 controls aged ⩾16 years were included in the case-control study. In total, 46/52 cases and 37/112 controls had consumed company X products [odds ratio (OR) 15·5, 95% confidence interval (CI) 6·1–39·7]. Of these, 36/46 cases and 16/37 controls had consumed pastries (OR 4·7, 95% CI 1·8–12·3), 25/46 cases and 12/37 controls had consumed filled doughnuts (OR 2·5, 95% CI 1·0–6·1). Sequence analysis of the VP1-2A junction region indicated 100% strain homology between cases and an infected employee of company X. We recommended reinforcement of hygiene precautions, and consideration of a prolongation of compulsory work absence after post-exposure vaccination.
INTRODUCTION
Background
In the last two decades, a substantial decrease in hepatitis A (HA) notifications has been observed in Germany. The notified incidence declined from 13·2/100 000 (East Germany) and 12·9/100 000 (West Germany) inhabitants in 1984 to 1·7/100 000 in the unified Federal Republic in 2003 (symptomatic and laboratory-confirmed or epidemiologically linked cases). In the 1998 National Health Survey, the overall seroprevalence of HA antibodies within the German population was estimated to be around 47% [1], and was shown to decrease with age: in the 70–79 years age group, 89% of the observed population had HA antibodies, compared with only 14% in the 18–29 years age group. The risk for outbreaks increases with decreasing seroprevalence in younger age groups. The declining immunity in younger persons in recent decades may have favoured outbreaks in several European countries [2–6].
In Germany, an average of 90 outbreaks per year were notified between 2001 and 2003. However, only few of them involved more than five cases; and food was infrequently the suspected vehicle of infection.
Onset of outbreak
Between 1 January and 6 April 2004, 28 cases of HA were notified to the local health authorities in two districts in southern North Rhine-Westphalia. An average of 11 cases per year had been notified in both districts combined during the previous 3 years. Further cases were notified in four surrounding districts, one of them in a neighbouring federal state. A first investigation by the local health authorities had already identified infections that had occurred during February in two members of family A, both employed in the bakery production line of the local food company X. One son of the family fell ill on 5 February and was diagnosed with HA. His mother, father and a brother who all also worked for company X received post-exposure vaccination on 9 February. As the mother worked as a vendor at a bakery store, she was asked to stay at home for 2 weeks after vaccination. The brother and father of the index patient both claimed to deal only with pre-baked products. Therefore, both were allowed to continue working. Despite the vaccination, the father fell ill on 27 February.
Food company X, a producer of meat and bakery products at two local production sites, sold its products in 27 branches including 19 bakery stores all of them within 50 km of the production site. In order to identify the extent of the outbreak, detect its source and the risk factors for infection, control the outbreak and make recommendations, an outbreak team consisting of members of Robert Koch-Institute (RKI) and the local health authorities of the two most affected districts conducted an epidemiological investigation.
METHODS
Case finding and descriptive epidemiology
We identified cases from notification records of six district health offices adjacent to company X. A press release was issued by the district public health authority in charge of the outbreak investigation in order to raise awareness among the local population. We checked the records of the major local laboratory in the most affected district in order to make sure all diagnosed cases had been notified.
We calculated the probable time of exposure by subtracting the longest possible incubation period (i.e. 50 days) from the last case and the shortest possible incubation time (i.e. 15 days) from the first case in the main wave of cases.
Case-control study
We performed a case-control study based on telephone interviews between 15 and 23 April. Cases were defined as persons resident in the six districts adjacent to company X in February 2005 with jaundice, fever, abdominal discomfort or elevated serum aminotransferase levels; with disease onset from 1 March to 8 April; and with positive serology for anti-HAV-IgM antibodies. Controls were defined as persons who had been staying in any of the six districts adjacent to company X in February 2005 without previous vaccination against HA or history of HA or jaundice since 1 March 2005. For legal reasons, we included only cases and controls aged ⩾16 years. For each case, we selected two control persons by random digit dialling. Telephone numbers were obtained by modifying the telephone numbers of cases with randomly generated two-digit numbers. We interviewed cases and controls using a standardized questionnaire including questions on consumption of:
bread, cakes and pastries (11 bakery products of company X);
raw meat products such as minced meat and raw sausages of company X or others;
between 18 and 28 February 2004, the most likely time of transmission.
In order to obtain a conservative estimate, all ‘don't know’ answers were counted as ‘no’ answers.
Data were entered into Epi-Info 2002 (CDC, Atlanta, GA, USA) for bivariate analysis to calculate odds ratios (ORs) and 95% confidence intervals (CIs). Results were tested by means of χ2 two-tailed P tests. We performed an unconditional logistic regression using a forward elimination procedure and maximum likelihood for significance testing in order to adjust the remaining significant exposure variable (filled doughnuts) for sex and age.
Laboratory
On 2 April, health authorities in the two most affected districts arranged testing of workers' stools in the two production sites of company X by polymerase chain reaction (PCR) to detect HAV-RNA. On 15 April, we performed a serological survey among the unaffected workers in the bakery department of company X.
The VP1-2A junction was amplified from cases' and HAV-IgM-positive employees' serum samples by nested PCR. All PCR products were sequenced.
Other methods
The outbreak team conducted an environmental inspection of company X, including bakery and meat production lines on 13 and 15 April. We observed working procedures with special regard to hygiene precautions taken and inspected toilets and washing facilities. Information about the means of transportation for bakery products and meat products was obtained.
RESULTS
Descriptive epidemiology
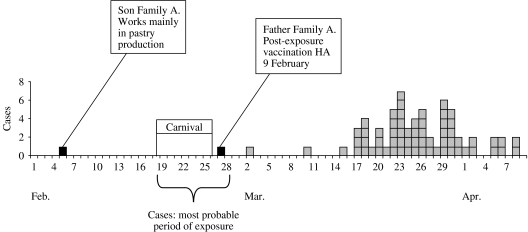
We identified 64 HA cases with disease onset between 1 March and 8 April. Most cases occurred between 17 March and 8 April (Fig. 1). The most probable time of exposure was calculated to be between 18 and 28 February 2004. This time period corresponded with the German carnival season.
Fig. 1.
Cases of hepatitis A by recorded onset of disease. North Rhine-Westphalia, Rhineland-Palatine, February–April 2004 (n=64). ■, Cases in company X employees;
 , cases.
, cases.
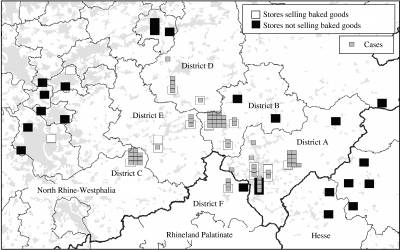
Cases were residents of five districts in North Rhine-Westphalia and one district in Rhineland-Palatinate. The spatial distribution of cases showed clustering of cases' places of residence around local bakery stores of company X (Fig. 2).
Fig. 2.
Cases of hepatitis A by place of residence and bakery stores selling company X's bakery goods. Densely populated areas marked in grey. North Rhine-Westphalia, Rhineland-Palatine, April 2004.
Thirty out of 64 cases were female and the median age was 40·5 years (range 6–80 years). In addition, four secondary cases were notified between 9 and 25 April, all close contacts of cases.
Based on the results of descriptive epidemiology and preliminary exploratory interviews, the hypothesis tested was: HA cases were more likely to have consumed bakery products of company X between 18 and 28 February than controls.
Analytical epidemiology: results of the case-control study
Fifty-two cases and 112 controls aged ⩾16 years from all the affected districts were eligible for the case-control study. Of the cases, 27 (52%) were female, and the median age was 43 years (range 16–80 years). Of the controls 72 (64%) were female and the median age was 45 years (range 17–82 years). In all, 46/52 cases (88·5%) and 37/112 controls (33·0%) had consumed company X products (OR 15·5, 95% CI 6·1–39·7). Among the subgroup of company X customers, cases were more likely than controls to have consumed any pastry: 36/46 cases (78·3%) compared with 16/37 controls (43·2%) (OR 4·7, 95% CI 1·8–12·3) (Table). Furthermore, 25/46 cases and 12/37 controls had consumed filled doughnuts (OR 2·5, 95% CI 1·0–6·1). Cases were less likely than controls to have eaten uncooked meat products (OR 0·3, 95% CI 0·1–0·7).
Table.
Results of bivariate analysis
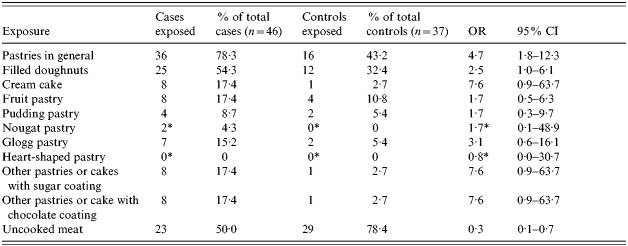
OR, Odds ratio; CI, confidence interval.
Odds of exposure (18–28 February) for cases of hepatitis A to various food items in the subgroup of company X consumers. Associations found remain significant when bivariate analysis is performed over all age groups and the total number of all consumers. North Rhine-Westphalia, Rhineland-Palatine, April 2004.
After introduction of fudge factor=1.
After adjusting for age and sex in a logistic regression model, consumption of doughnuts remained positively associated with disease (OR 3·6, 95% CI 1·3–9·8).
Laboratory
Serum samples for sequencing were obtained from 27 cases and two employees of company X (both members of family A). In samples from 22 out of 27 cases and 1 out of 2 employees (the father who had fallen ill) HAV genome could be amplified and subsequently sequenced. The sequence pattern of all HAV-RNA-positive samples indicated 100% sequence homology and belonged to HA subtype 1a. HAV-RNA was detected 47 days after onset of symptoms in one of the serum samples.
In the serological survey of the (asymptomatic) 18 employees of the bakery department of company X, only one serum sample was found anti-HAV-IgM positive. This person was another member of family A, who had received post-exposure vaccination on 9 February and had not been clinically ill. HAV genome could not be detected in his serum sample: the IgM was probably due to the active immunization against HA, although a subclinical infection cannot completely be excluded. In another five employees, anti-HAV-IgG was found, and in at least one of them a previous vaccination against HA was reported. The PCR for HAV was negative in all the stool samples submitted by all food handlers employed at company X.
Environmental inspection of company X
Company X produces meat and bakery products at the same location. However, the production lines are spatially separated, in two different parts of the building. Transportation of meat and bakery products to the selling points was carried out using the same delivery van. Although theoretically it might be possible that a worker contaminated the meat products, which subsequently contaminated the bakery products, this is quite unlikely, as bakery and meat products were always separated by fixed stable partitions in the vans.
The bakery production line was in the old part of the building. Due to spatial constraints, bread and fine bakery were produced in the same area. Pastries were glazed and packed for delivery in an area not spatially separated from the remaining production sites such as the bread ovens. Although bakery workers had designated tasks, it seemed from the inspection that, for efficiency, a clear separation of tasks, i.e. between working in the bread bakery and pastry bakery, was not usual. Bakery workers wore gloves on some occasions when glazing or icing pastries. Grip-free soap-and-disinfectant providers were available outside the toilet facilities of the bakery. The melting temperature for iced sugar used for coating filled doughnuts and other pastries was reported to be around 40°C but this could not be verified. In a separate room, the doughnuts were filled with a fruit jam before icing. This jam was delivered in closed buckets and provided by another food company. Members of family A did not work in this separated area and doughnuts were filled here by part-time workers who were employed during carnival season only. However, the family did work in a non-separated area where bread, cakes and pastries were baked and pastries, including doughnuts were coated at the same time. The father of family A, the bakery headman, assisted his colleagues in many production steps, when needed. He was, therefore, much more likely to have contact with bread and pastries. The source of infection of the first case in family A (the son of the bakery headman) was unknown.
DISCUSSION
This outbreak was one of the largest of HA in Germany in persons without travel history. It underlined the increasing risk of HA associated with a continuing decline of naturally acquired immunity in the population. Considering that asymptomatic infections especially occur in children, who were quite likely to have consumed contaminated doughnuts, we have to assume that more persons were infected.
The distribution of cases over time suggested a common source of infection. The suspected vehicles, based on the results of a case-control study and environmental inspections were filled doughnuts from company X. We considered that contamination of the products between 18 and 28 February by the second infected employee of company X (the father of family A) was most probable. This was supported by the descriptive epidemiology and the viral genome analysis. However, the second son of family A might have had subclinical HAV infection despite his vaccination and may also have been the source of this outbreak.
Our study has some limitations. Due to the long incubation period of HA and rumours spreading about the company, recall bias cannot be excluded. Only 25 (50%) of the cases in the case control-study can directly be explained by the consumption of pastries of company X. The remaining 10 company X consumers could have consumed such pastries whilst lacking active recall. Additionally, persons who claim not to have consumed products of company X might well have done so without knowing it, especially as during the carnival celebrations the consumption of doughnuts is very popular. As all ‘don't know’ answers had been counted as ‘no’ answers, the results of univariate analysis rather underestimate the associations found between illness and food exposure.
Transmission of HA viruses through food is favoured by their stability and the large numbers of virus excreted late in the incubation period before onset of clinical symptoms. Common source outbreaks have been attributed to various kinds of food contaminated by infected food handlers. Contaminated bakery products have been implicated previously in HA outbreaks. In 1976, a bakery worker finishing the sugar icing of doughnuts was probably the source of a HA outbreak in the United States [7]. In 1990, an outbreak of HA with 50 cases was traced back to the consumption of bread and in 1994, 79 persons were infected by contaminated pastries in New York [8]. In 1996, in a German outbreak, contaminated pastries were the most likely vehicle of transmission [9]. All these outbreak investigations were solely based on epidemiological evidence; viral genome sequencing or direct virus diagnosis from contaminated food had not been performed. In our outbreak, we were not able to identify HA virus in suspected food either, as no contaminated food was left for direct laboratory confirmation. This was because pastry products are delivered and consumed while fresh. Moreover, reference sampling is not mandatory for bakery products, therefore, no samples were available for investigation.
During our environmental investigation we found most bakery workers in the pastry production were not wearing gloves, although this does not necessarily imply an elevated risk of virus transmission. A recent investigation showed that bacterial contamination of the palms of hands did not vary between gloved and non-gloved hands in food handlers. Moreover, wearing gloves might even prevent regular hand hygiene [10]. The most important preventive measure remains regular and appropriate hand hygiene, especially after visiting toilets. The most effective hand hygiene for non-enveloped virus such as HAV is physical removal with soap and tap water [11].
We found amplifiable HA RNA in the serum of patients up to 47 days after onset of symptoms. These findings are consistent with a study which was able to detect HA RNA up to 55 days after symptom onset [12] and underline the usefulness of attempting genome amplification in outbreak investigations even if sera can only be obtained several weeks after onset of symptoms. Viral genome sequencing showed a 100% strain homology between serum samples of 23 cases and one of the food handlers (the father) and was genetically different from strains that had been recently isolated during other outbreaks in Germany. Nevertheless, more information about HA genotype circulation in Germany is necessary in order to improve evidence drawn from molecular epidemiology.
Interestingly, the second member of family A (father) fell ill 18 days after his HA post-exposure vaccination. He was a family contact of the first case, his son. Whether this was due to vaccine being given too late or a delayed immune response cannot be determined. If vaccine is administered close to exposure, HAV transmission may be prevented. Especially for food handlers, it is important to be aware that if the exact time of exposure cannot be determined, transmission will not be prevented in every case. It has been shown that about 80% of post-exposure vaccinated household contacts developed IgG antibodies within 14 days after vaccination [13]. However, the case of the father of the index family indicates that prolonging the compulsory work absence for food handlers after vaccination for HA contact to at least 3 weeks needs consideration.
The usefulness of active immunization of seronegative food handlers against HA as a tool to prevent HA outbreaks needs discussion. It seems clear that the ideal preventive measure in order to avoid foodborne outbreaks are hygiene precautions such as hand disinfection. A compulsory vaccination for food handlers in Germany has not yet been recommended. Vaccination of food handlers is costly and might not be effective in the subgroup of part-time seasonal workers who usually are recruited on demand. Some might even argue that vaccination against HA might give the food handler a false sense of security and lead to lowered hygienic alertness. In a cost-benefit study from the United States, the HA vaccination of restaurant workers was only cost-saving when administered during HA epidemics and if the costs did not exceed $20 per employee [14].
As a result of this outbreak investigation, we recommended that company X evaluate hygiene precautions such as hand washing and skin disinfection and enforce their implementation. Furthermore, we recommended considering a prolongation of the compulsory work absence for food handlers after vaccination for HA contact to at least 3 weeks. In the context of a potentially increasing number of foodborne HA outbreaks in Germany, we recommend for future outbreak investigations that any food handled by humans be investigated as vehicles of transmission, and to increase the use of molecular typing in HA cases.
ACKNOWLEDGEMENTS
We thank all colleagues involved from the District Health Departments and the Institute of Public Health of North Rhine-Westphalia for kindly hosting the outbreak team, their logistical support and their dedicated participation in our case-control study. We also thank our colleagues in the Department for Infectious Disease Epidemiology and the Unit for Molecular Epidemiology at RKI.
DECLARATION OF INTEREST
None.
REFERENCES
- 1.Thierfelder W et al. Prevalence of markers for hepatitis A, B and C in the German population. Results of the National Health Interview and Examination Survey 1998. European Journal of Epidemiology. 2001;17:429–435. doi: 10.1023/a:1013792013184. [DOI] [PubMed] [Google Scholar]
- 2.Malfait P et al. An outbreak of hepatitis A in Puglia, Italy, 1996. Eurosurveillance. 1996;1:33–35. [PubMed] [Google Scholar]
- 3.Ang LH. Outbreak of hepatitis A in a special needs school in Kent: 1999. Communicable Disease and Public Health. 2000;3:139–140. [PubMed] [Google Scholar]
- 4.Sayers G et al. Hepatitis A outbreak in an institution. Irish Medical Journal. 1999;92:396–398. [PubMed] [Google Scholar]
- 5.Severo CA et al. An outbreak of hepatitis A in a French day-care center and efforts to combat it. European Journal of Epidemiology. 1997;13:139–144. doi: 10.1023/a:1007336609638. [DOI] [PubMed] [Google Scholar]
- 6.Mele A et al. Outbreak of hepatitis A in Trieste, Italy. Journal of Public Health Medicine. 1994;16:242–243. doi: 10.1093/oxfordjournals.pubmed.a042964. [DOI] [PubMed] [Google Scholar]
- 7.Schoenbaum SC, Baker O, Jezek Z. Common-source epidemic of hepatitis due to glazed and iced pastries. American Journal of Epidemiology. 1976;104:74–80. doi: 10.1093/oxfordjournals.aje.a112275. [DOI] [PubMed] [Google Scholar]
- 8.Weltman AC et al. An outbreak of hepatitis A associated with bakery, New York, 1994: the 1968 ‘West Branch, Michigan’ outbreak repeated. Epidemiology and Infection. 1996;117:333–341. doi: 10.1017/s0950268800001515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Becker B, Prömse B, Kramer J, Exner M. Transmission of pathogenic human viruses by foods: hepatitis A epidemic caused by baked goods in the Euskirchen district. Gesundheitswesen. 1996;58:339–340. [PubMed] [Google Scholar]
- 10.Lynch RA et al. A preliminary evaluation of the effect of glove use by food handlers in fast food restaurants. Journal of Food Protein. 2005;68:187–190. doi: 10.4315/0362-028x-68.1.187. [DOI] [PubMed] [Google Scholar]
- 11.Sickbert-Bennett EE et al. Comparative efficacy of hand hygiene agents in the reduction of bacteria and viruses. American Journal of Infection Control. 2005;33:67–77. doi: 10.1016/j.ajic.2004.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nainan OV et al. Hepatitis A molecular epidemiology in the United States, 1996–1997: sources of infection and implications of vaccination policy. Journal of Infectious Diseases. 2005;15(191):957–968. doi: 10.1086/427992. [DOI] [PubMed] [Google Scholar]
- 13.Sagliocca L et al. Efficacy of hepatitis A vaccine in prevention of secondary hepatitis A infection: a randomised trial. Lancet. 1999;353:1136–1139. doi: 10.1016/S0140-6736(98)08139-2. [DOI] [PubMed] [Google Scholar]
- 14.Meltzer IM et al. The economics of vaccinating restaurant workers against hepatitis A. Vaccine. 2001;19:2138–2145. doi: 10.1016/s0264-410x(00)00396-0. [DOI] [PubMed] [Google Scholar]