SUMMARY
Salmonella Mississippi infections are very common in Australia's island state – Tasmania – with an annual rate of 17 cases/100 000 population. A case-control study conducted during 2001–2002 found single variable associations with indirect exposure to many native animal species, untreated drinking water, travelling within the state, hand–mouth behaviours and contact with pet faeces. No associations were detected with farm animal or pet species or with any food. Indirect contact with native birds, untreated drinking water and travel within the state remained significant predictors of infection in the final model with population attributable fractions of 0·57 and 0·54 for native animals and untreated drinking water respectively. In Tasmania, Australian wildlife species are the likely reservoir for S. Mississippi, contaminating land and water environments. To decrease infection rates requires treatment of water supplies, particularly private rainwater collection systems and advising people to wash their hands after being outdoors and prior to eating.
INTRODUCTION
Salmonella infections are a major cause of intestinal illness throughout the world [1]. In Australia, an estimated 92 000 (95% credible interval 26 000–158 000) cases of salmonellosis occur in the community each year [2]. While the source of the majority of infections is largely unknown, 87% are thought to be transmitted from contaminated food [2]. In addition, Salmonella is one of the commonest causes of outbreaks in Australia, and was responsible for 35% (75/214) of all outbreaks in Australia in the 6 years 1995–2000 [3].
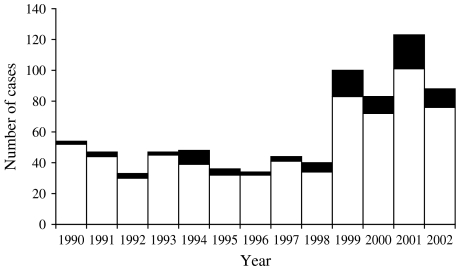
There are over 2000 distinct Salmonella serotypes that cause disease in humans [4]. However Salmonella Mississippi is an uncommon serotype in many parts of the world. In both Europe (I. Fisher, personal communication) and the United States [5] it accounts for <1% of all Salmonella infections. In Australia it is uncommon in all states except Tasmania, an island state with 2% of the Australian population. Approximately 80% of all S. Mississippi infections in the country are reported in Tasmanian residents (Fig. 1). Yet the overall rate of salmonellosis in Tasmania is less that for Australia as a whole (34·9 and 40·4/100 000 respectively in 2002). In 2002, Tasmania's rate of S. Mississippi infection was 17/100 000, which is remarkably high for a single serotype [6]. The environmental reservoir and vehicles of infection for this serotype remain unclear. There have been no major outbreaks of S. Mississippi infection since surveillance began and it has not been isolated during routine surveys of Tasmanian foods. Environmental surveys have highlighted that it is present in Tasmanian wildlife such as skinks, quolls (native cats) and kangaroos [7].
Fig. 1.
Human infections with S. Mississippi in Tasmania and other Australian States and Territories, 1990–2002 (Source: National Enteric Pathogen Surveillance Scheme, Microbiological Diagnostic Unit, University of Melbourne, Australia, 22 April 2005). □, Tasmania; ■, other Australian States and Territories.
To gain insight to the routes of transmission of S. Mississippi we conducted a case-control study in Tasmania from October 2001 to December 2002.
METHODS
Selection of cases
Cases of S. Mississippi were identified from the notifiable disease register held at the Tasmanian Department of Health and Human Services. An eligible case was defined as a laboratory-confirmed case of S. Mississippi infection reported between October 2001 and December 2002 with a recent history of acute diarrhoea. The study was extended beyond 12 months to accrue additional cases. Cases were excluded from the study if they lived in an institution, did not speak English well, or did not have a home telephone. Where household clusters occurred only the index case was included in the study.
The treating doctor was contacted prior to interview to ensure that the case had been informed of their diagnosis and to obtain consent to contact the case. Ethics approval was gained from the Royal Hobart Hospital Ethics Committee.
Selection of controls
Controls were recruited from an earlier population survey where subjects were randomly selected using true random digit dialling [8]. At the conclusion of the survey, the subject was asked whether members of their household would be willing to participate in future studies on health.
This study on S. Mississippi infection was undertaken in conjunction with a nationwide case-control study of Campylobacter infection. The two studies shared controls using identical questionnaires to capture risk factor information. Exclusion criteria for controls were similar to those used for cases, with controls also being excluded if they reported a history of diarrhoeal illness, campylobacteriosis, or salmonellosis in the month prior to interview.
During selection, controls were frequency matched to Campylobacter cases by age groups: 0–4, 5–9, 10–19, 20–29, 30–59, and ⩾60 years old. This resulted in a ratio of controls to S. Mississippi cases of approximately 4:1, with some imbalance in various age strata.
Interviews
Verbal consent was obtained from subjects prior to interview. A parent or guardian was interviewed if the case was <15 years of age. Cases aged 15–18 years were interviewed, subject to parental or guardian approval. Trained interviewers used a standardized questionnaire to collect information from cases and controls over the telephone. Cases were asked about exposures in the 7 days prior to the onset of diarrhoea while controls were questioned about exposures in the 7 days prior to interview.
Questionnaire
Data were collected on possible sources of exposure and included: foods consumed, drinking water sources, exposure to animals, recent travel, personal behaviours and demographic information. Indirect and direct exposure to specified native animals, farm livestock types and pets were measured using the questions ‘In those 7 days, were any of the following animals on the farm?’ and ‘Did you touch?’. For native animals, similar questions were asked, but related to the study subjects' environment rather than on the farm. Untreated water exposure was assessed both at home and outside the home. The food questions centred on runny eggs, food containing raw eggs, undercooked and barbequed meats, and the consumption of leftovers, raw fruits and vegetables.
A separate child questionnaire for subjects aged 0–4 years was administered to the person most familiar with the child's diet and habits. The child questionnaire limited food questions to those likely to be consumed by children <4 years old, and included breast- and bottle-feeding and additional behavioural characteristics.
Data analysis
We expressed exposure variables in dichotomous categories and used logistic regression to calculate crude odds ratios (ORs) with 95% confidence intervals (CIs). To take into account possible confounding, crude odds were adjusted for age dichotomized at 5 years. The selection of 5 years of age as the cut-off point was based on the distribution of cases and the change to the child questionnaire for children aged <5 years.
Following single variable analyses, we employed a two-stage modelling strategy. First, variables that were significantly associated with illness (P⩽0·1) in single variable analyses were allocated into the ‘exposure’ pathways that best reflected their potential routes of infection. Exposure pathways included: untreated water, native animal, farm animal or pet exposures, food consumption, behaviour, or location. For each of these exposure-pathway groupings a regression model was fitted, with age included in each model. Those variables that remained significant, independent predictors of illness in these single-exposure pathway models were retained for inclusion as candidate variables for the second stage, a final multiple-pathway model together with demographic characteristics representing potential confounders. The selection of candidate variables also considered biological plausibility, the accuracy of the exposure measure and the strength of association between covariates.
The final multiple-pathway model was constructed by backward elimination with dropped variables re-introduced one at a time. Biologically plausible interaction terms were examined. Population attributable fractions (PAF) for major risk factors were calculated using adjusted odds ratios presented in the final model.
Data were entered into a Microsoft Access database and analysed using stata version 8.0 (Stata Corporation, College Station, TX, USA).
RESULTS
In total, 89 cases of S. Mississippi infection were reported during the study period. Of these cases 71 were eligible to participate in the study and 83% (59/71) were enrolled. The reasons for non-participation included: being unable to contact the case, the doctor either unable to be contacted or advising that their patient should not be contacted, and a death due to other causes. No case refused to participate. Study participants were similar to cases not enrolled with respect to age, sex and regional distribution (data not shown).
The participation rate for controls was 75% with the principal reasons for non-participation being the inability to contact the control (n=62), followed by a refusal to participate (n=13).
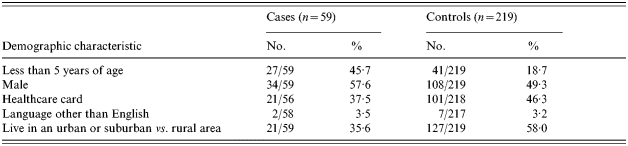
The case to control ratio varied across age strata with a ratio of 1:1 in the ages 0–3 years and increasing to 1:5·5 in subjects aged ⩾5 years. Cases were considerably younger than controls with 54% of cases aged <10 years, compared with only 26% of controls. This age imbalance was accounted for in the analysis. The gender distribution was similar with males comprising 58% of cases and 49% of controls (Table 1).
Table 1.
Demographic characteristics of S. Mississippi cases and population-based controls, Tasmania 2001–2002
Single variable analysis
Contact with animals
Information was sought on both indirect and direct exposure to animals. Because the number of cases and controls reporting direct contact with animals was very low, the results are not presented. Indirect exposure was measured through the reporting of the presence of native, farm or pet animals in environs of the case or control over a 7-day period.
Cases were more likely than controls to report exposure to environments where Australian native animals could be found. Elevated odds ratios were found for most native animal species when analysed separately and when treated as a composite variable. The highest risk estimates were found for quolls (native marsupial cats), native birds and skinks with crude odds ratios of 8·4, 4·9 and 4·4 respectively. Adjusting for age did not influence most risk estimates (Table 2).
Table 2.
Crude and age-adjusted univariate risks of S. Mississippi infection, Tasmania 2001–2002
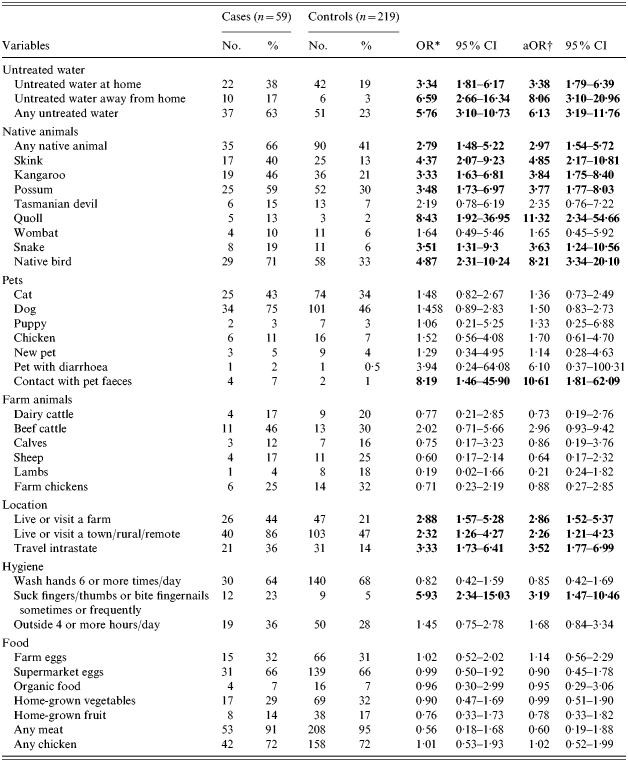
OR, Odds ratio; CI, confidence interval; aOR, adjusted odds ratio.
Odds ratios significantly associated with illness are highlighted in bold.
Odds ratios adjusted for age <5 years old.
Exposure to farm animals was not associated with illness. Cases were no more likely than controls to have been exposed to the environment of specific animals such as beef cattle, dairy cattle, sheep, pigs or chickens. Living or visiting a rural or remote area or small town, however, was associated with illness (OR 2·3). Cases were also more likely than controls to live on or visit a farm (OR 2·9) Overnight travel within the state was also associated with illness and largely consisted of travel to small towns, farms and natural areas.
Living in a household with either individual pet species or any pet was not associated with an increased risk. The risk estimates for having a new, young or ill pet in the home were also not elevated. However, recent exposure to pet faeces was associated with infection.
Untreated drinking water
Exposure to untreated drinking water at home and when visiting elsewhere were both significantly associated with illness, as was the combined variable. The risk estimate was greatest for exposure to untreated drinking water away from home (OR 6·6). Drinking-water risk estimates did not alter greatly after adjusting for age.
Food consumption
As 45% of cases were <5 years of age, the range of commonly eaten foods was limited, so all meats other than chicken were combined into one variable, and all chicken cuts included in the one variable. The undercooking of separate meats was combined into one variable, any undercooked meat or chicken. No association was detected with the consumption of any chicken, meat, eggs, or organic or home-grown fruit and vegetables. The consumption of unpasteurized milk was not associated with infection. Within the subset consisting of adults and children aged ⩾5 years, for whom more extensive dietary data were available, no associations were detected for variables that measured a variety of cuts of common meats as well as game and offal. Both the consumption of undercooked chicken and barbequed chicken were not associated with illness.
Other factors
Hand-to-mouth behaviour such as biting or chewing fingers, thumbs or fingernails (and for children <5 years of age, using a dummy) either every day or frequently was reported more often by cases than controls (OR 5·9). The effect estimate was higher for adults and children aged >5 years (OR 6·2) compared with children aged <5 years (OR 3·0). No associations were detected with other measures of general hygiene such as washing hands or with time spent in the outdoor environment, regardless of the level at which the exposure was dichotomized.
Multivariable model
Single-pathway models
Variables were allocated into one of the following four pathway models: animal exposure, untreated water exposure, location, and foods. When animal variables were entered into the one pathway model, exposure to an environment that included native birds remained a significant independent predictor. Both the broad measure of untreated water, which included both untreated water at home and away from home, and the subset, untreated water away from home, remained significantly associated with infection in the multivariable water exposure pathway model. As expected, the risk estimates for both of these measures were markedly less than the single variable risk estimates due to their close association with each other. Living in a small town, rural or remote area, time spent on a farm and travel within the state were all measures of location. When entered together in the one model, travel within the state remained a significant predictor of illness. For other exposure groupings only one variable was significant in single variable analyses and so multiple variable modelling was not necessary.
Multiple-pathway models
The variables that remained significant independent predictors of illness in each of the exposure pathway models included: exposure to an environment in which native birds were found, exposure to any untreated drinking untreated water and untreated drinking water away from home, living or visiting a farm, regular biting or chewing fingers, thumbs or fingernails or using a dummy, exposure to pet faeces and overnight travel within the state (Table 3). The final, most parsimonious model included untreated water, intra-state travel and exposure to a native bird environment. Age was also included in the model. Together these factors were able to explain 32% of the variance in the model. Neither the re-entry of excluded variables nor the addition of interaction terms improved the model.
Table 3.
Multiple variable logistic regression models of exposures associated with S. Mississippi infection, Tasmania 2001–2002
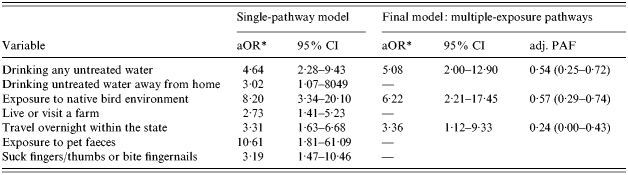
aOR, Adjusted odds ratio; CI, confidence interval; PAF, population-attributable fraction.
Odds ratios adjusted for other exposure variables in the model and age <5 years old.
High adjusted PAFs were found for exposure to untreated drinking water 0·54 (95% CI 0·25–0·72) and exposure to a native bird environment 0·57 (95% CI 0·29–0·74).
Two alternate modelling strategies were also employed in which either all variables that were significantly associated with illness (P⩽0·01) were entered into one model; or plausible composite variables were used where appropriate. While there was some change in the variables selected in the final model, all three models contained a variable that represented untreated drinking water and an Australian native animal exposure variable. Adjusted attributable factions for these exposure routes were consistent across all three modelling strategies.
DISCUSSION
The finding of strong single variable associations with many Australian native animal species and the retention of a native animal variable in the final model suggest the reservoir for S. Mississippi infection is to be found in the wildlife population. The lack of association of the same indirect measure of exposure for both farm and pet animals strengthen this hypothesis. These findings are supported by an earlier bacteriological survey of wildlife that isolated S. Mississippi from faecal samples of many native animals including skinks, snakes, quolls, Tasmanian devils and kangaroos [7]. A greater proportion of skinks and quolls were infected with S. Mississippi compared with other animal species and it was speculated that infection may originate from a reptile species such as the skink, which contaminates the local environment thereby infecting other animals.
Reptiles such as snakes, iguanas and lizards have been identified as a source of salmonellosis in outbreaks [9, 10] as well as sporadic cases of infection [11]. Our finding that direct contact with host animals was not necessary for infection is supported by others, for example, living or visiting a residence in which pet iguanas were held was associated with S. Marina infection [12].
Contamination of the outdoor environment by native animals has also been reported as a cause of infections in southern United States where living in areas where native amphibians are found was associated with S. Javiana infection [14].
In this study, indirect exposure to a native bird environment remained a significant independent predictor of illness in the multivariable model. There has been no bacteriological testing of birds to confirm this finding. It is plausible that birds are a reservoir of infection, especially as rainwater tanks were the source of untreated drinking water in 81% of cases. However, it must also be considered that native bird exposure may be a marker for exposure to a range of wildlife as 82% of subjects also reported exposure other native animal environments.
In Norway, outbreaks of human S. Typhimurium infection have been paralleled with the carriage of the same PFGE strains of S. Typhimurium in hedgehogs [15] and post-mortem records of Salmonella in birds, 1969–2000, also identified these strains [16]. The bacteriological findings were supported by a case-control study where exposure to wild birds or their droppings was a significant independent predictor of human infection [17]. Birds have also been implicated in the recent incursion of S. Typhimurium DT160 into the New Zealand population [18].
The distinctive seasonal and age distribution of S. Mississippi infection in Tasmania lends further support for an environmental route of transmission. The summer peak for S. Mississippi infection exceeds that of other serotypes with 86% of infections reported in the 6 months of warmer weather in 2002 [6]. The marked seasonal changes in the behaviour of both the host animal species and humans may account for the distribution pattern. Some native animal species, particularly reptiles, hibernate in winter potentially reducing the environmental load in these months, and at the end of the dry summer period native animals are likely to encroach upon urban, agricultural and recreational land seeking feed and water thereby contaminating the environment shared by humans over summer.
An animal reservoir may help explain the marked dominance of infections in children <5 years of age. During 2002, 46% of all reported S. Mississippi cases in Tasmania occurred in children <5 years of age compared with 15% for other Salmonella serotypes [6]. Young children are more likely to engage in hand-to-mouth activities, spend time close to the ground and are more susceptible to low-dose inoculum. Other serotypes widely regarded as acquired through environmental exposure, such as S. Ball and S. Birkenhead in Australia (J. Powling, personal communication, November 2004) and S. Typhimurium in Norway also show a similar age distribution [17].
In this study, the association between S. Mississippi infection and hand–mouth activities such as biting nails, chewing fingers/thumbs and dummy use differed by age; the association was strongest for adults and children aged ⩾5 years old, with 11% of cases and 2% of controls reporting these exposures. The association was less likely to be detected in the younger age group due to the commonness of these activities in young children.
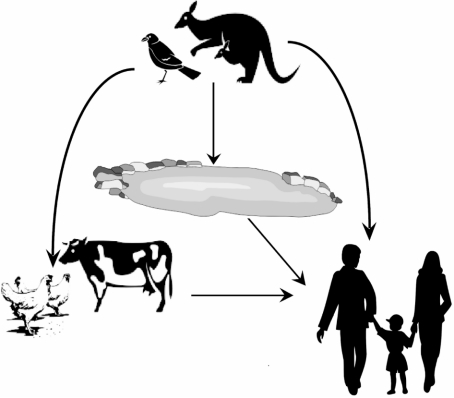
Both farm animals and pets may acquire infection and become intermediate hosts through the consumption of infected wildlife, contaminated soil or plant material. S. Mississippi has occasionally been isolated from both pets and livestock, and we observed elevated crude odds for contact with pet faeces. A proposed transmission chain for S. Mississippi in Tasmania is shown in Figure 2.
Fig. 2.
Possible transmission pathways for S. Mississippi infection in Tasmania, Australia.
We identified a significant association between cases of S. Mississippi infection and exposure to untreated drinking water, both at home and away from home. Most untreated water exposure was recorded as exposure to water collected in rainwater collection tanks. Risk estimates for exposure to rainwater tanks were significant (P<0·001) both for exposure away from home and any rainwater tank exposure. The literature records outbreaks of salmonellosis associated with drinking contaminated water [19, 20]. Our findings for sporadic disease are concordant with a Norwegian case-control study that detected similar associations between a wildlife reservoir, untreated water and salmonellosis due to a specific Salmonella serotype [17].
Risk estimates were higher when untreated water exposure was limited to occasions spent away from home and may reflect a lower level of immunity in populations not frequently exposed to S. Mississippi.
No cases reported interstate or overseas travel, however, 36% of cases reported overnight travel within the state. The majority of these cases (67%) resided in urban or suburban areas with 93% of these urban cases travelling to rural areas. Thus, it is conceivable that travel within the state is a marker for exposure to a natural environment. It is unlikely that associations with common foods were undetected as Tasmania exports foods to other Australian states and few cases are reported in other regions. Of cases reported in other states, the majority report recent travel to Tasmania [6].
Biases in case-control studies are always of concern, however, many of the possible biases in this study would result in an underestimate of effect. Prior immunity in the control population could mean that some controls were exposed to S. Mississippi and were asymptomatic cases at the time of the study. Such misclassification of controls would bias associations towards the null. Moderate degrees of prior immunity may reduce risk estimates in outbreaks of waterborne pathogens [21]. Similarly, the collection of exposure data over a 7-day period, which is beyond the usual 1–3 day incubation period for salmonellosis could reduce the strength of the associations found [22]. To reduce recall bias, controls were asked about the 7 days preceding the interview rather than the matching 7-day period for cases. Salmonella infection is commonly regarded as a foodborne illness, so it was anticipated that questions relating to water and animal exposures would not be subject to significant knowledge bias.
In summary, this study illustrates that indirect exposure to a variety of native animals confers a risk of infection that is supported by bacteriological findings. While we were unable to discriminate between native animal reservoir species and animal hosts, the public health message regarding hand washing following time spent outdoors, particularly in a natural environment remains consistent. Exposure to untreated drinking water was also a strong independent predictor of infection with the principal source of untreated drinking water being rainwater collection tanks. While S. Mississippi has occasionally been isolated from Tasmanian river water, further bacteriological surveys are required to confirm that untreated drinking water is contaminated and that the elevated risk estimate is not because drinking water is a surrogate for another unrecognized exposure.
Currently S. Mississippi occupies an environmental niche, which has potential to spill over into the food supply. S. Mississippi has occasionally been isolated from both waterways and livestock and vigilance is required to prevent any movement into these pathways. With a high environmental load in Tasmania this may be difficult to detect, making it is essential to fully investigate cases occurring in other Australian jurisdictions to identify any emerging sources of infection.
ACKNOWLEDGEMENTS
We thank David Coleman, Vanessa Madden, Jo-Ann Payne and Denise Bassett, and diagnostic and reference laboratories for their contributions to this study. David Jordan and Mark Veitch provided helpful comments on a draft of this manuscript. Alison Milton prepared the transmission pathway figure. The Australian Government Department of Health and Ageing provided funding for this project under the OzFoodNet program of work.
DECLARATION OF INTEREST
None.
REFERENCES
- 1.Hohman EL. Nontyphoidal salmonellosis. Clinical Infectious Diseases. 2001;32:263–269. doi: 10.1086/318457. [DOI] [PubMed] [Google Scholar]
- 2.Hall G et althe OzFoodNet Working Group. Estimating foodborne gastroenteritis, Australia. Emerging Infectious Diseases. 2005;11:1257–1264. doi: 10.3201/eid1108.041367. . and . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dalton C et al. Foodborne disease outbreaks in Australia, 1995–2000. Communicable Diseases Intelligence. 2004;28:211–224. [PubMed] [Google Scholar]
- 4.Humphrey T. Salmonella, stress responses and food safety. Nature Reviews Microbiology. 2004;2:504–509. doi: 10.1038/nrmicro907. [DOI] [PubMed] [Google Scholar]
- 5.CDC. Salmonella surveillance: annual summary 2000. Atlanta, GA: US Department of Health and Human Services; 2001. [Google Scholar]
- 6.OzFoodNet Working Group. Foodborne disease in Australia: incidence, notifications and outbreaks. Annual report of the OzFoodNet network, 2002. Communicable Diseases Intelligence. 2003;27:209–243. [PubMed] [Google Scholar]
- 7.Ball A. The epidemiology of salmonella serovars in Tasmania. M.Sc. Dissertation, University of Tasmania, 1991 [Google Scholar]
- 8.Hall GV et al. the OzFoodNet Working Group. Frequency of infectious gastrointestinal illness in Australia, 2002: regional seasonal and demographic variation. Epidemiology and Infection. 2006;134:111–118. doi: 10.1017/S0950268805004656. and . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Friedman CR et al. An outbreak of salmonellosis among children attending a reptile exhibit at a zoo. Journal of Pediatrics. 1998;132:802–807. doi: 10.1016/s0022-3476(98)70307-5. [DOI] [PubMed] [Google Scholar]
- 10.CDC. Outbreaks of multidrug-resistant Salmonella Typhimurium associated with veterinary facilities – Idaho, Minnesota, and Washington, 1999. Morbidity and Mortality Weekly Report. 2001;30:701–704. [PubMed] [Google Scholar]
- 11.CDC. Reptile associated salmonellosis – selected States, 1998–2202. Morbidity and Mortality Weekly Report. 2003;52:1206–1209. [PubMed] [Google Scholar]
- 12.Mermin J, Hoar B, Angulo F. Iguanas and Salmonella Marina infection in children: a reflection of the increasing incidence of reptile-associated salmonellosis in the United States. Pediatrics. 1997;99:399–402. doi: 10.1542/peds.99.3.399. [DOI] [PubMed] [Google Scholar]
- 13.Mermin J et al. Reptiles, amphibians, and human Salmonella infection: a population-based, case-control study. Clinical Infectious Diseases 2004. 38:253–261. doi: 10.1086/381594. (Suppl 3): [DOI] [PubMed] [Google Scholar]
- 14.Srikantiah P et al. Salmonella enterica serotype Javiana infections associated with amphibian contact, Mississippi, 2001. Epidemiology and Infection. 2004;132:273–281. doi: 10.1017/s0950268803001638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Handelhand K et al. Prevalence of Salmonella Typhimurium infection in Norwegian hedgehog populations associated with two human disease outbreaks. Epidemiology and Infection 2002. 128:523–527. doi: 10.1017/s0950268802007021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Refsum T et al. Salmonellae in avian wildlife in Norway from 1969 to 2000. Applied and Environmental Microbiology. 2002;68:5595–5599. doi: 10.1128/AEM.68.11.5595-5599.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kapperud G, Stenwig H, Lassen J. Epidemiology of Salmonella Typhimurium O: 4–12 infection in Norway. Evidence of transmission from an avian wildlife reservoir. American Journal of Epidemiology. 1998;147:774–782. doi: 10.1093/oxfordjournals.aje.a009522. [DOI] [PubMed] [Google Scholar]
- 18.Thornley CN et al. First incursion of Salmonella enterica serotype Typhimurium DT160 into New Zealand. Emerging Infectious Diseases. 2003;9:493–495. doi: 10.3201/eid0904.020439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Angulo FJ et al. A community waterborne outbreak of salmonellosis and the effectiveness of a boil water alert. American Journal of Public Health. 1997;87:580–584. doi: 10.2105/ajph.87.4.580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Taylor R et al. A waterborne outbreak of Salmonella Saintpaul. Communicable Diseases Intelligence. 2000;24:336–340. [PubMed] [Google Scholar]
- 21.Hunter PR. Modelling the impact of prior immunity, case misclassification and bias in case-control studies in the investigation of outbreaks of cyrptosporidiosis. Epidemiology and Infection. 2000;125:713–718. doi: 10.1017/s0950268800004854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Molbak K, Niemann J. Risk factors for sporadic infection with Salmonella enteritidis, Denmark, 1997–1999. American Journal of Epidemiology. 2002;156:654–661. doi: 10.1093/aje/kwf096. [DOI] [PubMed] [Google Scholar]