SUMMARY
The aim of this study is to assess the effects of immigration from countries with a high prevalence of tuberculosis (HPCs), of HIV/AIDS prevalence, and the ageing of the indigenous population, on tuberculosis distribution in a low-prevalence area (LPCs), the Piedmont Region of Italy. Tuberculosis incidence and HIV cases were identified by linking records from the surveillance systems. Overall, 640 tuberculosis cases were identified and crude annual incidence was found to be 17·3/100 000. The incidence rate ratio for HIV infection as a risk factor for tuberculosis (11·4 and 51·9 among individuals from HPCs and LPCs respectively) was greater than that for immigration from HPCs (6·7 and 30·9 among HIV+ and HIV− individuals). Immigration accounted for a larger number of incident cases [population attributable risk % (PAR %): 31·8 and 52·8% among HIV+ and HIV− individuals] than did HIV infection (PAR %: 5·4 and 11·1% among individuals from HPCs and LPCs). Efforts should be made to identify and treat young immigrants from HPCs.
INTRODUCTION
The epidemiology of tuberculosis (TB) in low-prevalence countries (LPCs) is strongly influenced by interdependent host-related factors such as immigration from high-prevalence countries (HPCs), the distribution of HIV/AIDS in the population and the ageing of the indigenous population [1, 2]. The role of each of these factors must be considered in relation to the distribution of the others and of the population mixing patterns. Recent immigration from HPCs to industrialized countries usually involves young adults [3], whereas the resident population of LPCs is steadily ageing and their background risk of developing TB is progressively declining. Individuals in certain low-income areas of the world (e.g. sub-Saharan Africa, south Asia and eastern Europe) are at higher risk of both HIV and Mycobacterium tuberculosis infection [4, 5].
Although several studies designed to estimate the fraction of TB cases attributable to HIV infection [5–7] and to immigration from HPCs [3, 8] have been carried out, the distribution, interplay and impacts of the two factors remain largely unknown, hampering public health policies for TB prevention and control [9, 10]. Accurate estimates of the impacts of HIV and immigration on TB incidence are essential for designing appropriate, targeted strategies for TB eradication in LPCs. To our knowledge the issue has never been investigated in European industrialized areas experiencing recent immigration from HPCs, such as Italy or Spain. We, therefore, investigated the role of HIV infection and immigration from HPCs on TB incidence in a region of Italy where, as in many other areas in western Europe [10, 11], immigration from HPCs, particularly Africa and eastern Europe, is rising steadily [12, 13]. Previously published data from the Piedmont Region and Turin (the regional capital) underscore the roles of HIV infection, which reached a peak of 16·5% among TB cases during 1994–1996, and immigration, accounting for 25·8% of all TB cases in 1997–1999, in shaping TB epidemiology [14, 15]. In order to assess the role of both factors on TB incidence, we carried out a population-based study on incident TB cases in the Piedmont Region in 2001.
METHODS
The study base was the entire resident adult population of the Piedmont Region in 2001, subdivided by age (15–49 and ⩾50 years), sex and geographical origin (individuals born in the Piedmont Region to immigrants from high-risk countries were classified as being from a low-risk country). Data on the resident population were obtained from the 14th national census [16], performed the same year (Table 1). About one-third of immigrants were from Africa, one-third from Eastern Europe or the former Soviet Union, and the remainder from Asia and Latin America. The countries of origin of the investigated population were divided into two groups, as advised by the Italian Ministry of Health: HPCs (estimated incidence ⩾50 cases/100 000 population) and LPCs (estimated incidence <50 cases/100 000 population). The latter group includes Italy [4, 17]. For the general immigrant population, reliable information on the date of immigration was not available, whereas for TB cases information was incomplete and of questionable quality, therefore, we were unable to categorize immigrants on the basis of the time since immigration.
Table 1.
Adult population of Piedmont, Italy, 2001, and distribution of cases of tuberculosis (TB), by age group, geographical origin and HIV status

The investigation focused on new TB cases, incident in 2001 in the region. TB cases were identified by deterministic record-linkage between four institutional sources: the TB Notification Registry, the TB Treatment Outcome Monitoring System, the Laboratory TB Register and hospital discharge records. After correction for duplicate entries in the four registers, the records of the TB cases were matched by full name, sex and date of birth. All apparent matches were reviewed to avoid homonymous and synonymous errors. TB cases, diagnosed in 2000, were identified and were not included in the study, whereas cases found to be incident in 2001 were corrected for late reporting in the first half of 2002. A case verification procedure to confirm the diagnosis was performed by inspecting the hospital discharge records of patients identified solely from this source. TB cases were defined as follows: confirmed (culture-confirmed or smear-positive) cases due to Mycobacterium tuberculosis complex (M. tuberculosis, M. bovis and M. africanum) and probable cases (clinically, radiologically or empirically diagnosed), according to the European framework for TB control and elimination in countries with a low incidence [18]. For each identified case, we collected information on sex, age, country of origin, site of infection and TB case definition (microbiologically confirmed or unconfirmed). Similarly, HIV/AIDS cases were identified from the regional HIV/AIDS regional surveillance system. The HIV and TB surveillance systems cover the same population.
In Italy, there is no national surveillance system for HIV infection, and the only available data are derived from systems organized on a regional basis. The Piedmont HIV surveillance system, activated in 1999, is based on clinical records in the 14 regional centres entitled to deliver antiretroviral treatment; these are the only centres in the region that are allowed to manage HIV cases. Moreover, all HIV cases treated outside the region are reported to the regional authorities for reimbursement purposes. Although notification of HIV cases is not mandatory, nonetheless all the regional centres actively participate in the notification process. The HIV surveillance system is considered to be reasonably complete for patients who have had at least one contact with one of the regional centres. Cases in untested individuals and in those who have never contacted one of the centres cannot, by definition, be detected. The regional surveillance system collects information on age, sex, country of birth, possible risk behaviour for acquiring HIV infection and some clinical information on people living with HIV/AIDS. The patients' identities are encrypted to maintain case uniqueness while making personal identification impossible. During an evaluation exercise by the national AIDS registry, the probability of generating the same code for different individuals was estimated to be 1·3/1000 (Istituto Superiore di Sanità – COA, unpublished data). Through deterministic record-linkage, by linking identical codes with the aforementioned encrypted coding system to maintain patient confidentiality, we merged data from the regional HIV/AIDS surveillance system and the TB dataset.
We calculated the sex-standardized (standard: Piedmont's resident population) annual TB incidence rates, stratified by age classes, HIV status and geographical origin. We fitted a Poisson regression model to estimate the sex-adjusted, age-stratified incidence rate ratios (IRRs) and the fraction of new TB cases attributable to HIV infection and immigration from HPCs as percentage population attributable risk (PAR %) [19]. The statistical analyses were carried out with the stata version 8.2 software package (StataCorp., College Station, TX, USA).
RESULTS
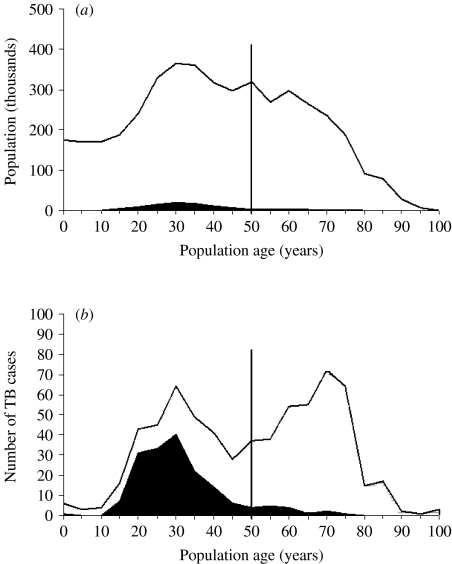
Through record-linkage, we identified and included 640 adult cases of incident TB in 2001. During the same year there were 5382 patients living with HIV in Piedmont, which means an HIV prevalence of 0·14%, with 0·13% among people from LPCs and 0·52% among those from HPCs. Table 1 summarizes the distribution of Piedmont residents and TB cases, stratified by age group, geographical origin and HIV status. The Figure (a, b) shows the age distribution according to geographical origin, of Piedmont residents and TB cases respectively. Overall, it is possible to appreciate the effect of age and geographical origin of these individuals on TB incidence. Of the 168 cases of TB in immigrants, 43 (25%) were in people from Eastern Europe or the former Soviet Union, 89 (52%) in people from Africa, 20 (12%) in people from Latin America and 13 (8%) in people from Asia. The country of origin was unknown for three cases.
Fig.
Stacked area graph showing (a) the age distribution of the resident population and (b) the age distribution of tuberculosis (TB) cases by geographical area of origin in Piedmont, Italy, 2001. □, Individuals from countries with low prevalence of TB; ■, individuals from countries with high prevalence of TB.
The overall crude annual TB incidence rate in 2001 was 17·3 cases/100 000 population. Table 2 shows the sex-standardized annual incidence rates stratified by age class, geographical origin and HIV status. The annual incidence rates among people <50 years old ranged from 3·3 cases/100 000 population for HIV-negative patients from LPCs to 1139·5 cases/100 000 population for HIV-positive patients from HPCs. The annual TB incidence among elderly people from LPCs was 2–3 times higher than among younger persons, regardless of HIV status. No significant age-related differences in TB incidence were found for HIV-negative persons from HPCs; however, no estimates could be made for elderly HIV-positive persons from HPCs as no cases of TB were observed in this group.
Table 2.
Estimated sex-standardized annual incidence rates of tuberculosis (TB) in Piedmont, Italy, 2001 (per 100 000 persons per year), stratified by age group, geographical origin and HIV status

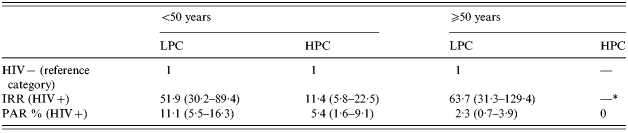
The sex-adjusted IRRs and the corresponding sex-adjusted PAR %, measuring the relative and absolute effects of HIV infection and immigration from HPCs are shown in Tables 3 and 4 respectively. HIV-positive status (Table 3) appeared to promote TB among those from LPCs, regardless of age, and among people from HPCs. The corresponding estimates of sex-adjusted PAR % showed that the impact of HIV infection on TB incidence among younger individuals in the Piedmont population is ∼11% for people from LPCs and ∼5·4% for those from HPCs. For people >50 years old, the IRR and PAR % could be estimated only for those from LPCs (63·7 and 2·3% respectively), as no HIV-positive immigrants from HPCs were identified in this age group.
Table 3.
Sex-adjusted measures of effect (incidence rate ratio, IRR) and impact (population attributable risk percentage, PAR %) of HIV infection on incidence of tuberculosis (TB) in Piedmont, Italy, 2001, stratified by age and geographical origin
LPC, Low TB prevalence countries; HPC, high TB prevalence countries.
No cases of tuberculosis in this group.
Table 4.
Sex-adjusted measurements of effect (incidence rate ratio, IRR) and impact (population attributable risk percentage, PAR %) of geographical origin on incidence of tuberculosis (TB) in Piedmont, Italy, 2001, stratified by age and HIV infection status
LPC, Low TB prevalence countries; HPC, high TB prevalence countries.
No cases of tuberculosis in this group.
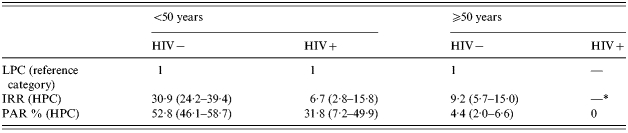
The effect of immigration from HPCs (Table 4) on annual TB incidence among HIV-negative immigrants is smaller among those >50 years than among their younger counterparts (IRR 30·9 and 9·2 respectively). As we did not identify any HIV-positive immigrant >50 years, we observed no analogous difference among HIV-positive HPC immigrants. The corresponding estimates of the impact of immigration from HPCs on TB incidence among younger people gave a PAR % of 52·8% for HIV-negatives and 31·8% for HIV-positives. For elderly HIV-negative people, the PAR % was ∼4·4%. Thus, immigration from HPCs accounted for a larger number of incident cases than did HIV infection even though the relative risk was smaller.
The combined effect of HIV infection and immigration from HPCs on annual TB incidence, estimated only for people <50 years, was smaller than would be expected if the two exposures were acting independently. More specifically, the effect of HIV-positive status decreased the IRR from 51·9 for persons from LPCs to 11·4 for immigrants from HPCs (Table 3). Similarly, the effect of immigration from HPCs decreased the IRR from 30·9 for HIV-negative people to 6·7 for HIV-positive people (Table 4). These risk reductions are paralleled by corresponding drops in impact estimates, the PAR % decreasing from 11·1 to 5·4% for HIV infection and from 52·8 to 31·8% for immigration from HPCs.
Age did not appear to influence the effect of HIV infection (Table 3) on the development of TB in people from LPCs, whereas it showed some influence on the effect of immigration from HPCs among HIV-negative individuals (Table 4). The IRR was 30·9 for people from 15 to 50 years old, whereas it was 9·2 for those >50 years.
DISCUSSION
Several studies have explored the effects on TB incidence of the distribution of HIV infection in developing and industrialized countries and of immigration from HPCs to LPCs [20]. As far as we know, however, the concomitant roles of immigration and HIV have not been directly addressed. In this study, we assessed the roles of immigration from HPCs, HIV infection and the ageing of the indigenous population on TB incidence in the Piedmont Region in 2001. The geographical position and socio-economic features of this area, which are similar to those elsewhere in industrialized Western Europe, particularly to those areas experiencing analogous patterns of immigration from HPCs, make the results of this investigation valuable for the assessment and design of targeted prevention and control strategies focused on TB transmission.
These estimates might have been affected by both a possible lack of accuracy of the 14th national census and an unknown number of undetected cases of TB and HIV infection, in particular when considering the highly mobile social group of illegal immigrants. Overall, this may result in an underestimation of the effects of both HIV and immigration. The proportion of illegal immigrants in the overall immigrant population was estimated in 1999 to be <20% and it has probably fallen in recent years [21].
The incidence estimates show that the annual risk of developing TB in the Piedmont community varies widely depending on the population subset considered. The occurrence of higher incidence rates among aged persons in LPCs [22] is not observed among those from HPCs, who are rarely >65 years (representing only ∼3% of all immigrants from HPCs). The variation in the age distribution of people >50 years (Fig.) may well explain the discrepancy in the age effect between the two geographically defined groups. Indeed, a higher risk of latent TB infection reactivation has been recorded among people who are >65 years [2].
The annual incidence rates estimated for younger immigrants from HPCs, both HIV-negatives (102·7 cases/100 000 population) and HIV-positives (1139·5 cases/100 000 population), are similar to those observed in several HPCs [4]. The effect of immigration on TB rates is reduced among elderly individuals >50 years, possibly as a consequence of the longer residency in LPCs of elderly foreign-born people. Duration of residency in LPCs is known to be associated with a progressive reduction in the risk for TB [23].
Role of immigration on annual risk for TB
The role of immigration from HPCs in TB prevention and control in our region is a key topic, in 2001, as many as 53% of the observed cases among young HIV-negatives and 32% of those among HIV-positives were in immigrants. The proportion of immigrants from HPCs among incident cases of TB (26%, 168/640) is consistent with that observed in 2001 in France (35%) [24] and with model-based estimates for the Dutch population (12% in 2000 to 22% in 2010) [3] but smaller than that estimated for the United Kingdom (63%) [25]. Since our definition of HPCs encompasses areas with different levels of TB prevalence, the latter difference may be explained by dissimilar patterns of immigration occurring in different European countries.
The impact of immigration from HPCs is likely to increase in the future as active disease continues to develop in young immigrants with latent TB infection, unless major efforts are mounted to screen for infection and provide treatment. Indeed, the pool of immigrants from HPCs, which accounted for only 2% of the regional adult population in 2001 (Fig.), has subsequently nearly doubled from 95 872 in 2001 to 167 615 in 2003 – and is expected to grow further in the next few years [12].
Role of HIV infection on annual risk for TB
The overall prevalence of HIV-positivity among the TB cases in our study was 5% (32/640), while that for the total Piedmont population is ∼0·14%. Consequently, although HIV infection is the single most important factor in promoting TB infection progression [26] and plays a major role in shaping TB epidemics in HPCs [27], the fraction of cases in Piedmont attributable to HIV infection is ∼11%. Our data thus confirm that HIV infection contributes moderately to overall TB morbidity in industrialized areas [7], mainly because of the narrow overlap between TB-infected and HIV-infected population segments. When considering the effect of HIV infection and immigration on TB incidence it is important to take into account the incidence of HIV in the source populations along with the immigration pattern specific to a particular area. In particular, it can be expected that the role of HIV may become more relevant in the future, should a larger proportion of cases with TB-HIV co-infection move to the Piedmont Region.
Interestingly, HIV infection and immigration from HPCs, occurring simultaneously only in people <50 years, do not combine as independent events. The ‘healthy immigrant effect’ [28] is a reasonable explanation of the reduced effect of HIV infection among immigrants (Tables 3 and 4). HIV infection acquired before or after immigration might be less advanced in people who need to be healthier to emigrate and to bear the consequences of immigration and are consequently less immune-compromised and less prone to develop TB.
In conclusion, we argue that currently in the Piedmont area, the HIV and TB epidemics are striking different population sectors [26, 29–31]. Therefore, in TB eradication [31], major efforts should be devoted to the identification and treatment of TB and latent infection in young immigrants from HPCs [9].
ACKNOWLEDGEMENTS
The authors thank Professor Benedetto Terracini, Professor Neil Pearce and Dr Lorenzo Richiardi for helpful support and enlightening comments on the research, and Susan Phillips, English reader at the Turin University, for the language revision. The CRC methods were developed within the framework of the Special Project ‘Oncology’, Compagnia San Paolo FIRMS and by a grant from the Italian Association for Cancer Research. The study was supported by ‘Ricerca Finalizzata’ Regione Piemonte/CIPE.
DECLARATION OF INTEREST
None.
REFERENCES
- 1.Broekmans J, Reichman L, Hershfield E. Tuberculosis: A Comprehensive International Approach. 2nd edn. New York: Dekker; 2000. Tuberculosis control in low prevalence countries; pp. 75–92. , pp. [Google Scholar]
- 2.Rajagopalan S. Tuberculosis and aging: a global health problem. Clinical Infectious Diseases. 2001;33:1034–1039. doi: 10.1086/322671. [DOI] [PubMed] [Google Scholar]
- 3.Wolleswinkel-van BJ et al. The impact of immigration on the elimination of tuberculosis in The Netherlands: a model based approach. International Journal of Tuberculosis and Lung Disease. 2002;6:130–136. [PubMed] [Google Scholar]
- 4.World Health Organization Geneva, Switzerland: 2005. . Global tuberculosis control: surveillance, planning, financing. WHO Report, , 2005. [Google Scholar]
- 5.Corbett EL. HIV and tuberculosis: surveillance revisited. International Journal of Tuberculosis and Lung Disease. 2003;7:709. [PubMed] [Google Scholar]
- 6.Aisu T et al. Preventive chemotherapy for HIV-associated tuberculosis in Uganda: an operational assessment at a voluntary counselling and testing centre. AIDS. 1995;9:267–273. [PubMed] [Google Scholar]
- 7.Rose AM et al. An estimate of the contribution of HIV infection to the recent rise in tuberculosis in England and Wales. Thorax. 2002;57:442–445. doi: 10.1136/thorax.57.5.442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lillebaek T et al. Risk of Mycobacterium tuberculosis transmission in a low-incidence country due to immigration from high-incidence areas. Journal of Clinical Microbiology. 2001;39:855–861. doi: 10.1128/JCM.39.3.855-861.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Coker R. Compulsory screening of immigrants for tuberculosis and HIV. British Medical Journal. 2004;328:298–300. doi: 10.1136/bmj.328.7435.298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Verver S, van Soolingen D, Borgdorff MW. Effect of screening of immigrants on tuberculosis transmission. International Journal of Tuberculosis and Lung Disease. 2002;6:121–129. [PubMed] [Google Scholar]
- 11.Lillebaek T et al. Persistent high incidence of tuberculosis in immigrants in a low-incidence country. Emerging Infectious Diseases. 2002;8:679–684. doi: 10.3201/eid0807.010482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ciardelli L 2004. . The presence of foreign-born people in statistics of the inter-institutional observatory on foreign-born people in Turin Province. 2003 Report [in Italian]. Turin: ISTAT – Ufficio territoriale per il Piemonte e la Valle d'Aosta – Sede di Torino. Divisione Servizi Civici, Ufficio di Statistica,
- 13.El-Hamad I et al. Screening for tuberculosis and latent tuberculosis infection among undocumented immigrants at an unspecialised health service unit. International Journal of Tuberculosis and Lung Disease. 2001;5:712–716. [PubMed] [Google Scholar]
- 14.Matteelli A et al. Supervised preventive therapy for latent tuberculosis infection in illegal immigrants in Italy. American Journal of Respiratory and Critical Care Medicine. 2000;162:1653–1655. doi: 10.1164/ajrccm.162.5.9912062. [DOI] [PubMed] [Google Scholar]
- 15.Faggiano F et al. Tuberculosis incidence in Turin, Italy, 1973–1999. International Journal of Tuberculosis and Lung Disease. 2004;8:171–179. [PubMed] [Google Scholar]
- 16.Decreto Del Presidente Del Consiglio Dei Ministri. The legal population of the Republic according to the census of 21 October 2001 [in Italian]. Gazzetta Ufficiale Serie Generale 2003; N. 81 (Suppl. Ordinario n. 54).
- 17.Ministry of Health. Guidelines for the control of tuberculosis, Ministry of Health proposal [in Italian]. Supplemento ordinario n. 35 alla G.U. n. 40 del 18 febbraio 1999. Serie generale.
- 18.Borgdorff MW, Floyd K, Broekmans JF. Interventions to reduce tuberculosis mortality and transmission in low- and middle-income countries. Bulletin of the World Health Organization. 2002;80:217–227. [PMC free article] [PubMed] [Google Scholar]
- 19.Greenland S, Drescher K. Maximum likelihood estimation of the attributable fraction from logistic models. Biometrics. 1993;49:865–872. [PubMed] [Google Scholar]
- 20.Clancy L et al. Tuberculosis elimination in the countries of Europe and other industrialized countries. European Respiratory Journal. 1991;4:1288–1295. [PubMed] [Google Scholar]
- 21.Censis. Report on the Social status of the country – 1999 [in Italian]. Milano: F. Angeli, 1999.
- 22.McDonald R, Reichman L, Hershfield E. Tuberculosis: A Comprehensive International Approach. New York: Dekker; 1993. Tuberculosis in the elderly; pp. 413–439. , pp. [Google Scholar]
- 23.McKenna MT, McCray E, Onorato I. The epidemiology of tuberculosis among foreign-born persons in the United States, 1986 to 1993. New England Journal of Medicine. 1995;332:1071–1076. doi: 10.1056/NEJM199504203321606. [DOI] [PubMed] [Google Scholar]
- 24.Anon. Tuberculosis and migrants [in French] Revue des maladies respiratoires. 2003;20:S68–S69. [PubMed] [Google Scholar]
- 25.Mohammad A, Clare F, Delphine A London: 2004. . Annual report on tuberculosis cases reported in 2001 in England, Wales and Northern Ireland. : Tuberculosis Section, Communicable Disease Surveillance Centre, Health Protection Agency, [Google Scholar]
- 26.Selwyn PA et al. A prospective study of the risk of tuberculosis among intravenous drug users with human immunodeficiency virus infection. New England Journal of Medicine. 1989;320:545–550. doi: 10.1056/NEJM198903023200901. [DOI] [PubMed] [Google Scholar]
- 27.Crampin AC et al. Tuberculosis and gender: exploring the patterns in a case control study in Malawi. International Journal of Tuberculosis and Lung Disease. 2004;8:194–203. [PubMed] [Google Scholar]
- 28.McDonald JT, Kennedy S. Insights into the ‘healthy immigrant effect’: health status and health service use of immigrants to Canada. Social Science and Medicine. 2004;59:1613–1627. doi: 10.1016/j.socscimed.2004.02.004. [DOI] [PubMed] [Google Scholar]
- 29.Burwen DR et al. National trends in the concurrence of tuberculosis and acquired immunodeficiency syndrome. Archives of Internal Medicine. 1995;155:1281–1286. [PubMed] [Google Scholar]
- 30.Hamers FF, Downs AM. The changing face of the HIV epidemic in western Europe: what are the implications for public health policies? Lancet. 2004;364:83–94. doi: 10.1016/S0140-6736(04)16594-X. [DOI] [PubMed] [Google Scholar]
- 31.Rieder HL et al. Surveillance of tuberculosis in Europe. Working Group of the World Health Organization (WHO) and the European Region of the International Union Against Tuberculosis and Lung Disease (IUATLD) for uniform reporting on tuberculosis cases. European Respiratory Journal. 1996;9:1097–1104. doi: 10.1183/09031936.96.09051097. [DOI] [PubMed] [Google Scholar]