SUMMARY
A nationwide study was undertaken to determine the susceptibility to penicillin and serotypes of Streptococcus pneumoniae in Japan. S. pneumoniae was isolated from 114 adult patients with community-acquired pneumonia over 22 months at 20 hospitals and medical centres in different regions in Japan. All but five isolates were from sputum. Forty-eight isolates (42·1%) were susceptible, 40 (35·1%) showed intermediate resistance (MIC, 0·12–1·0 μg/ml) and 26 (22·8%) were resistant (MIC, ⩾2·0 μg/ml) to penicillin G. All isolates were susceptible to ceftriaxone (breakpoint 1 μg/ml), imipenem (4 μg/ml) and vancomycin (4 μg/ml). Most were resistant to erythromycin, clarithromycin and azithromycin; only two were resistant to levofloxacin. Differences were found in the distribution of serotypes among isolates showing susceptibility to penicillin (predominant types 3, 6B, and 19F), intermediate resistance (6B, 14, 19F, and 23F) and full resistance (19F and 23F). PFGE typing showed that 14 of the 25 strains of serotype 19F had a single DNA profile, pattern A, a pattern closely similar to that of the Taiwan multidrug-resistant 19F clone. Twelve pattern A strains were not susceptible to penicillin but carried the macrolide resistance gene mef(A). The DNA profiles of the 15 strains of 23F were also heterogeneous but six were highly similar (pattern b) yet distinct from the Spanish multidrug-resistant 23F clone although possibly related to the Taiwan multidrug-resistant 23F clone. The pattern b strains were not susceptible to penicillin and also harboured either mef(A) or erm(B). Our results indicate that multidrug-resistant pneumococci are spreading rapidly in Japan. Efforts to prevent the spread of the pandemic multidrug-resistant serotypes should be intensified.
INTRODUCTION
Streptococcus pneumoniae is the major cause of community-acquired pneumonia (CAP) as well as otitis media, sinusitis, septicaemia, and meningitis [1]. Although susceptible to penicillin in the past, penicillin-resistant and multidrug-resistant pneumococci are now widespread in the world [1, 2]. In Japan, S. pneumoniae is the most common cause of CAP [3], and the proportion of multidrug-resistant strains is increasing at a dramatic rate [4, 5]. Nevertheless, there are few reports on the distribution of various serotypes and the genetic relatedness of pneumococci in this country [6]. In this study, we describe the results of a nationwide survey of the antimicrobial susceptibilities and the distribution of pneumococcal serotypes isolated from patients with CAP. We also examined the genetic relatedness of the predominant multidrug-resistant serotype 19F and 23F strains by pulsed-field gel electrophoresis (PFGE) and compared their DNA profiles with selected pandemic reference strains.
METHODS
Bacterial strains
A total of 114 isolates of pneumococci were recovered from 114 adult patients (mean age 67·4, range 20–99 years) presenting with CAP between November 2001 and August 2003 at 20 hospitals and medical centres located in different regions of Japan. Pneumonia was diagnosed if there was an appearance of a new abnormal shadow and likely infiltration on a chest roentgenogram and if at least two of the following clinical and laboratory findings were present: fever (temperature>37·8°C), cough, production of purulent sputum, dyspnoea, and leukocytosis (WBC count>10 000/μl). The sources of the isolates were sputum (n=109), blood (n=3), pleural effusion (n=1) and bronchoalveolar lavage (n=1). Culture plates were incubated overnight in a 5% CO2 atmosphere, and optochin sensitivity and bile solubility tests were performed to confirm S. pneumoniae.
Antimicrobial susceptibility test
MICs were determined by an agar dilution method according to the guidelines of the National Committee for Clinical Laboratory Standards [7]. All isolates were tested for susceptibility to the following nine antibiotics: penicillin G (Meiji Seika Kaisha, Tokyo, Japan), ceftriaxone (Chugai Pharmaceutical Co., Tokyo, Japan), cefditoren (Meiji Seika Kaisha), imipenem (Banyu Pharmaceutical Co., Tokyo, Japan), erythromycin (Dainippon Pharmaceutical Co., Osaka, Japan), clarithromycin (Taisho Pharmaceutical Co., Tokyo, Japan), azithromycin (Pfizer Japan Inc., Tokyo, Japan), levofloxacin (Daiichi Pharmaceutical Co., Tokyo, Japan), and vancomycin (Shionogi Co., Osaka, Japan).
Serotyping
Isolates were serotyped by the capsular swelling (quellung reaction) observed microscopically after suspension in pneumococcal typing antisera (Statens Serum Institut, Copenhagen, Denmark).
PCR
PCR was performed to detect alterations in penicillin-binding protein genes pbp1a, pbp2x, and pbp2b and macrolide resistance genes mef(A) and erm(B) by using a commercially available test kit (Wakunaga Pharmaceutical Co., Hiroshima, Japan) with primers modified as reported previously [8]. Briefly, a single colony from the blood agar medium was suspended in a microtube containing 30 μl of a lysis solution. The tube was placed in a thermal cycler, and bacterial cells were lysed at 60°C for 10 min followed by 94°C for 5 min. Next, 2 μl lysate was placed in a PCR tube containing 25 μl reaction mixture (1 ml reaction mixture contained 60 ng of a primer for each of the target genes, 80 μl of 10 mm dinucleoside triphosphate, 40 U Tth DNA polymerase, and 100 μl of 10× PCR buffer). The PCR conditions were 94°C for 20 s, 52°C for 20 s, and 72°C for 15 s for 30 cycles total.
PFGE
PFGE was performed on strains of serotype 19F, 23F. The Spanish multidrug-resistant serotype 23F clone (ATCC 700669), the Taiwan multidrug-resistant serotype 19F clone (ATCC 700905) and the Taiwan multidrug-resistant serotype 23F clone (ATCC 700906) were used as reference standards [9]. Strains were grown overnight in brain heart infusion broth at 35°C, and PFGE of SmaI chromosomal digests was performed as described previously [10]. DNA banding patterns were interpreted according to the criteria of Tenover et al. [11] with a greater than three bands difference in profile needed to distinguish between PFGE types.
RESULTS
Antimicrobial susceptibility test
The MICs of the nine antibiotics tested for the 114 S. pneumoniae isolates are shown in Table 1. Using NCCLS breakpoints all isolates were found to be susceptible to ceftriaxone, imipenem and vancomycin. The majority were resistant to erythromycin, clarithromycin and azithromycin; only two isolates were not susceptible to levofloxacin. Forty-eight isolates (42·1%) were susceptible to penicillin G, 40 (35·1%) showed intermediate resistance and 26 (22·8%) were fully resistant to ⩾2·0 μg/ml of this antibiotic.
Table 1.
Distribution of MICs for various antibiotics for 114 isolates of pneumococci in Japan
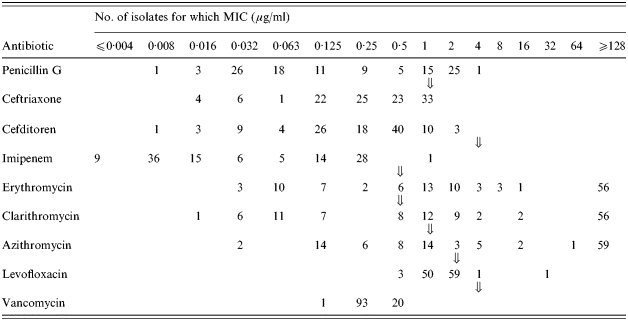
Breakpoints for each antibiotic.
Serotyping
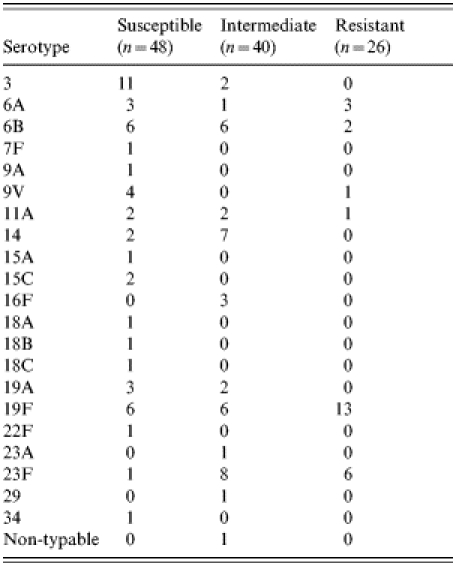
All isolates but one, were grouped into 21 different serotypes (Table 2). The serotypes of penicillin-susceptible isolates varied widely but with serotypes 3 (22·9%), 6B (12·5%) and 19F (12·5%) predominating among 18 other serotypes. In contrast, the 26 fully penicillin-resistant isolates fell into only six serotypes, the most frequent being 19F (50·0%), and 23F (23·1%); intermediate susceptible isolates were represented by 11 serotypes with 23F (20·0%), 14 (17·5%), 6B (15·0%) and 19F (15·0%) being the most common.
Table 2.
Serotypes of susceptible, intermediate, and fully penicillin-resistant pneumococci
Molecular characterization of serotypes 19F and 23F
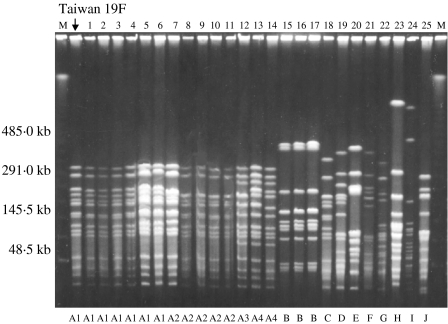
PFGE typing revealed 10 different DNA profiles (A–J) among the 25 strains of serotype 19F (Fig. 1). One pattern, type A, accounted for 14 strains and this was indistinguishable from the profile of the Taiwan multidrug-resistant serotype 19F clone. Three strains of serotype 19F were classified as pattern B and the other eight strains gave unique patterns. Twelve of the pattern A strains were not susceptible to penicillin and all 14 harboured the mef(A) gene. Overall 21 (84%) of the serotype 19F strains were mef(A) and/or erm(B) positive. Eighteen of the serotype 19F strains had alterations in pbp1a, −2x, and −2b; four in pbp2x and −2b; two in pbp2x; and one had no alteration. These strains originated from different geographical regions of Japan (Table 3).
Fig. 1.
Pulsed-field gel electrophoresis (PFGE) patterns of SmaI-digested DNA from 25 strains of serotype 19F S. pneumoniae and a representative of the Taiwan multiresistant serotype 19F clone. M, Molecular size marker.
Table 3.
Correlation of PFGE patterns, penicillin resistance, macrolide resistance genes and mutation in penicillin binding protein genes in strains of S. pneumoniae serotypes 19F and 23F
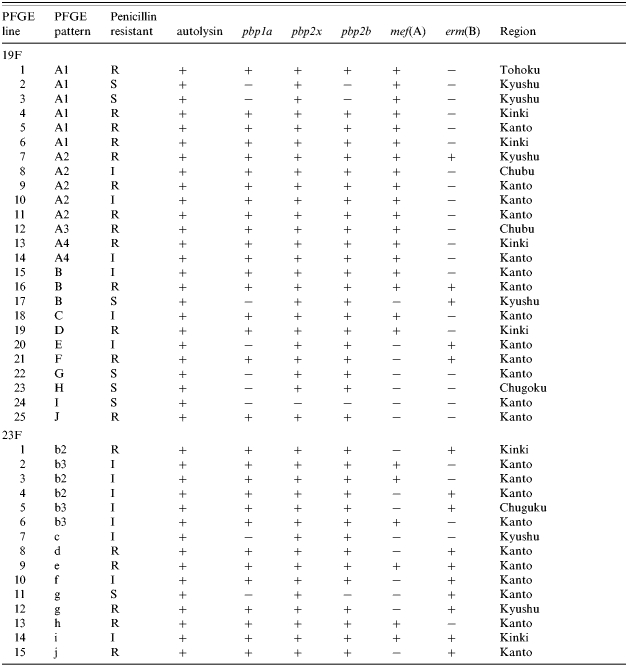
S, penicillin-susceptible. I, penicillin intermediate resistant. R, penicillin-resistant.
Penicillin binding protein genes pbp1a, pbp2x, and pbp2a: +, altered; −, not altered.
Macrolide resistant genes mef(A) and erm(B): +, positive; −, negative.
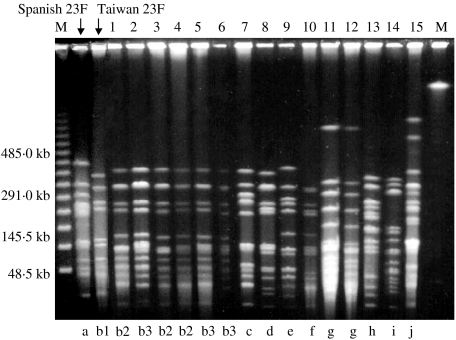
The 15 strains of serotype 23F were characterized by nine different DNA profiles (b–j) with pattern b predominating and represented by six strains (Fig. 2). Two strains were typed as pattern g and the remainder were unique. None of the DNA profiles of serotype 23F matched that of the Spanish multidrug-resistant 23F clone but pattern b was similar to the profile of the Taiwan multidrug-resistant serotype 23F clone although there were at least four band differences between them (Fig. 2). Strains of pattern b were not susceptible to penicillin and all positive by PCR for either the mef(A) or erm(B) gene as were strains of the other DNA types. These strains were also widespread in the country (Table 3). Thirteen of the serotype 23F strains were altered in pbp1a, −2x, and −2b; and one each in pbp2x and −2b, and pbp2x respectively.
Fig. 2.
Pulsed-field gel electrophoresis (PFGE) patterns of SmaI-digested DNA from 15 strains of serotype 23F S. pneumoniae and representatives of the Spanish and Taiwan multiresistant serotype 23F clone. M, Molecular size marker.
Two strains each of serotype 19F (patterns A2 and B) and 23F (patterns e and i) had both mef(A) and erm(B) genes, and these four strains were all penicillin non-susceptible (Table 3).
DISCUSSION
Penicillin-resistant S. pneumoniae are already distributed worldwide and resistance appears to be expanding to include multiple antimicrobial agents [1, 4]. In Asia, the proportion of S. pneumoniae reported to be non-susceptible to penicillin over the last decade ranges from 68·8% in Thailand [12], to 3·8% in India, 9·0% in Malaysia, 9·8% in China, 21·0% in Indonesia, 23·1% in Singapore, 38·7% in Taiwan, 41·2% in Sri Lanka, 60·8% in Vietnam, 79·7% in Korea and 65·3% in Japan in 1996 to 1997 [13]. Indeed, a survey of a single prefecture in Japan from 2001 to 2003 reported a frequency of 60·9% [6]. In this study, 57·9% of the S. pneumoniae isolates from patients with CAP were penicillin non-susceptible and some of these were also resistant to various other antibiotics. Thus, it appears that the frequency of multidrug-resistant S. pneumoniae in Japan is among the highest in Asia. A previous study demonstrated that the aetiology of CAP in Japan did not differ substantially from Western countries and that S. pneumoniae was the most common pathogen in this disease [3]. Some earlier surveys indicate that the clinical outcome between penicillin-susceptible and non-susceptible isolates does not differ under current resistance levels [5, 14]. However, invasive pneumococcal isolates with very high-level penicillin resistance (MIC⩾8·0 μg/ml) have been reported to be prevalent in the United States [15]. Caution should be exercised concerning such strains, although we did not detect them in Japan.
The 23-valent pneumococcal polysaccharide vaccine (PS23) has been promoted worldwide, although it fails to protect children <2 years old [16, 17]. It is well known that children aged <5 years and adults of ⩾65 years are the most susceptible to invasive pneumococcal infection and have a high mortality rate [16, 18]. Vaccination, therefore, seems to be the only available method for preventing pneumococcal disease [16, 19]. In this study, all the isolates were classified into 21 serotypes, 82·5% of which are accounted for in PS23. Furthermore, 56 (59·6%) of the isolates were penicillin non-susceptible isolates. Although the efficacy of PS23 against pneumonia without bacteraemia is controversial [20], a combination of influenza and pneumococcal vaccination appears to be beneficial for the reduction of mortality from all causes in individuals aged ⩾65 years [21].
In the group of serotype 23F pneumococci, six out of 15 strains showed the predominant PFGE pattern b which was different from the pattern of the Spanish multidrug-resistant 23F clone but similar to the Taiwan multidrug-resistant serotype 23F clone. Although a previous study indicated that the Spanish multidrug-resistant 23F clone was spreading in Asia [13], it was not detected in this survey. On the other hand, 14 out of 25 strains of serotype 19F showed the predominant pattern A which was closely related to the pattern for the Taiwan multidrug-resistant 19F clone. Since these predominant serotypes were collected from different regions in Japan and most were penicillin non-susceptible with multiple alterations in pbp and all isolates had macrolide resistant genes, these results suggest that derivatives of the Taiwan multidrug-resistant 19F and 23F clones have the potential to spread further in Japan. Recently, it was reported by Kasahara et al. [6] that these clones had already spread in Japan. However, theirs was a pilot study and they recognized the need for an urgent nationwide surveillance for these strains. Our nationwide study focusing on CAP-associated pneumococci has yielded results consistent with their earlier findings.
Moreover, it has been suggested in a recent report that pneumococcal isolates containing both the mef(A) and erm(B) genes may have originated from the Taiwan multidrug-resistant serotype 19F clone containing the mef(A) gene after introduction of the erm(B) gene [22]. However, one serotype 19F strain different from the Taiwan serotype 19F clone and two serotype 23F strains containing both the mef(A) and erm(B) genes were identified in our study suggesting that other types of antibiotic-resistant pneumococci might appear in Japan.
In conclusion, our results indicate that multidrug-resistant pneumococci are spreading rapidly in Japan and that efforts to prevent spread of pandemic multidrug-resistant serotype 19F and 23F clones should be intensified.
ACKNOWLEDGEMENTS
We thank all the staff in participating institutions of this study: Department of Internal Medicine, Graduate School of Medicine, Tohoku University (5 patients), Shiogama Municipal Hospital (2 patients), Department of Respirology, Graduate School of Medicine, Chiba University (4 patients), Funabashi Municipal Medical Center (1 patient), Chiba Municipal Kaihin Hospital (1 patient), Department of Respiratory Medicine, Juntendo University School of Medicine (5 patients), Fourth Department of Internal Medicine, Nippon Medical School (4 patients), First Department of Internal Medicine, Kyorin University, School of Medicine (26 patients), National Organization Tokyo Hospital (20 patients), National Center for Geriatrics and Gerontology (2 patients), Nagoya Ekisaikai Hospital (1 patient), Anjo Kosei Hospital (3 patients), Aichi Prefectural Showa Hospital (3 patients), First Department of Internal Medicine, Osaka Medical College (8 patients), Minoh Municipal Hospital (6 patients), Department of Respiratory Medicine, Kawasaki Medical University (3 patients), Kyorin Hospital (7 patients), Tagami Hospital (2 patients), Nagasaki Medical Center of Neurology (9 patients), Takasugi Clinic (2 patients). We also thank Banyu Pharmaceutical Co., Tokyo, Japan for help in completing this study. We also thank Akihiro Wada (Department of Bacteriology, Institute of Tropical Medicine, Nagasaki University), Chieko Shimauchi (Miyazaki Prefectural Nursing University) and Matsuhisa Inoue (Kitasato University School of Medicine) for their help in completing the PFGE studies. Thanks are also due to Kei Kasahara (Second Department of Internal Medicine, Nara Medical University) for providing the Taiwan multidrug-resistant serotype 23F clone.
This study was supported by a Grant-in-Aid for U.S.–Japan Cooperative Medical Science Program, Acute Respiratory Infections Panel.
DECLARATION OF INTEREST
None.
REFERENCES
- 1.Appelbaum PC. Antimicrobial resistance in Streptococcus pneumoniae: an overview. Clinical Infectious Diseases. 1992;15:77–83. doi: 10.1093/clinids/15.1.77. [DOI] [PubMed] [Google Scholar]
- 2.Linares J et al. Trends in antimicrobial resistance of clinical isolates of Streptococcus pneumoniae in Bellvitge Hospital, Barcelona, Spain (1979–1990) Clinical Infectious Diseases. 1992;15:99–105. doi: 10.1093/clinids/15.1.99. [DOI] [PubMed] [Google Scholar]
- 3.Ishida T et al. Etiology of community-acquired pneumonia in hospitalized patients: a 3-year prospective study in Japan. Chest. 1998;114:1588–1593. doi: 10.1378/chest.114.6.1588. [DOI] [PubMed] [Google Scholar]
- 4.Rikitomi N et al. Rapid increase of pneumococcal resistance to β-lactam and other antibiotics in isolates from the respiratory tract (Nagasaki, Japan: 1975–1994) Microbiology and Immunology. 1996;40:899–905. doi: 10.1111/j.1348-0421.1996.tb01158.x. [DOI] [PubMed] [Google Scholar]
- 5.Watanabe H et al. A comparative clinical study of pneumonia by penicillin-resistant and -sensitive Streptococcus pneumoniae in a community hospital. Respirology. 2000;5:59–64. doi: 10.1046/j.1440-1843.2000.00227.x. [DOI] [PubMed] [Google Scholar]
- 6.Kasahara K et al. Clonal dissemination of macrolide-resistant and penicillin-susceptible serotype 3 and penicillin-resistant Taiwan 19F-14 and 23F-15 Streptococcus pneumoniae isolates in Japan: a pilot surveillance study. Journal of Clinical Microbiology. 2005;43:1640–1645. doi: 10.1128/JCM.43.4.1640-1645.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.NCCLS National Committee for Clinical Laboratory Standards; Wayne, PA: 1998. . Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically. Approved standard M7–A4. [Google Scholar]
- 8.Ubukata K, Iwata S, Sunakawa K. In vitro activities of new ketolide, telithromycin, and eight other macrolide antibiotics against Streptococcus pneumoniae having mefA and ermB genes that mediate macrolide resistance. Journal of Infection and Chemotherapy. 2003;9:221–226. doi: 10.1007/s10156-003-0258-2. [DOI] [PubMed] [Google Scholar]
- 9.McGee L et al. Nomenclature of major antimicrobial-resistant clones of Streptococcus pneumoniae defined by the Pneumococcal Molecular Epidemiology Network. Journal of Clinical Microbiology. 2001;39:2565–2571. doi: 10.1128/JCM.39.7.2565-2571.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yano H et al. Pulsed-field gel electrophoresis analysis of nasopharyngeal flora in children attending a day care center. Journal of Clinical Microbiology. 2000;38:625–629. doi: 10.1128/jcm.38.2.625-629.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tenover FC et al. Interpreting chromosomal DNA restriction patterns produced by pulsed-field gel electrophoresis: criteria for bacterial strain typing. Journal of Clinical Microbiology. 1995;33:2233–2239. doi: 10.1128/jcm.33.9.2233-2239.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Watanabe H et al. Antimicrobial susceptibility and serotype distribution of Streptococcus pneumoniae and molecular characterization of multidrug-resistant serotype 19F, 6B, and 23F pneumococci in North Thailand. Journal of Clinical Microbiology. 2003;41:4178–4183. doi: 10.1128/JCM.41.9.4178-4183.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Song JH et al. Spread of drug-resistant Streptococcus pneumoniae in Asian countries: Asian Network for Surveillance of Resistant Pathogens (ANSORP) study. Clinical Infectious Diseases. 1999;28:1206–1211. doi: 10.1086/514783. [DOI] [PubMed] [Google Scholar]
- 14.Aspa J et al. Drug-resistant pneumococcal pneumonia: clinical relevance and related factors. Clinical Infectious Diseases. 2004;38:787–798. doi: 10.1086/381886. [DOI] [PubMed] [Google Scholar]
- 15.Schrag SJ et al. Emergence of Streptococcus pneumoniae with very-high-level resistance to penicillin. Antimicrobial Agents and Chemotherapy. 2004;48:3016–3023. doi: 10.1128/AAC.48.8.3016-3023.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bogaert D et al. Pneumococcal vaccines: an update on current strategies. Vaccine. 2004;22:2209–2220. doi: 10.1016/j.vaccine.2003.11.038. [DOI] [PubMed] [Google Scholar]
- 17.U.S. Centers for Disease Control. Prevention of pneumococcal disease: recommendations of the Advisory Committee on Immunization Practices (ACIP) Morbidity and Mortality Weekly Report. 1997;46 (RR-8):1–24. [PubMed] [Google Scholar]
- 18.Mantese OC et al. Prevalence of serotypes and antimicrobial resistance of invasive strains of Streptococcus pneumoniae. Journal of Pediatrics. 2003;79:537–542. [PubMed] [Google Scholar]
- 19.Martens P et al. Serotype-specific mortality from invasive Streptococcus pneumoniae disease revisited. BMC Infectious Diseases. 2004;4:21. doi: 10.1186/1471-2334-4-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mangtani P, Cutts F, Hall AJ. Efficacy of polysaccharide pneumococcal vaccine in adults in more developed countries: the state of the evidence. Lancet Infectious Diseases. 2003;3:71–78. doi: 10.1016/s1473-3099(03)00514-0. [DOI] [PubMed] [Google Scholar]
- 21.Christenson B et al. Effects of a large-scale intervention with influenza and 23-valent pneumococcal vaccines in adults aged 65 years or older: a prospective study. Lancet. 2001;357:1008–1011. doi: 10.1016/S0140-6736(00)04237-9. [DOI] [PubMed] [Google Scholar]
- 22.Ko KS, Song JH. Evolution of erythromycin-resistant Streptococcus pneumoniae from Asian countries that contains erm(B) and mef(A) genes. Journal of Infectious Diseases. 2004;190:739–747. doi: 10.1086/422156. [DOI] [PubMed] [Google Scholar]