SUMMARY
A case-control study was undertaken in an acute district general hospital to identify risk factors for hospital-acquired bacteraemia caused by methicillin-resistant Staphylococcus aureus (MRSA). Cases of hospital-acquired MRSA bacteraemia were defined as consecutive patients from whom MRSA was isolated from a blood sample taken on the third or subsequent day after admission. Controls were randomly selected from patients admitted to the hospital over the same time period with a length of stay of more than 2 days who did not have bacteraemia. Data on 42 of the 46 cases of hospital-acquired bacteraemia and 90 of the 92 controls were available for analysis. There were no significant differences in the age or sex of cases and controls. After adjusting for confounding factors, insertion of a central line [adjusted odds ratio (aOR) 35·3, 95% confidence interval (CI) 3·8–325·5] or urinary catheter (aOR 37·1, 95% CI 7·1–193·2) during the admission, and surgical site infection (aOR 4·3, 95% CI 1·2–14·6) all remained independent risk factors for MRSA bacteraemia. The adjusted population attributable fraction, showed that 51% of hospital-acquired MRSA bacteraemia cases were attributable to a urinary catheter, 39% to a central line, and 16% to a surgical site infection. In the United Kingdom, measures to reduce the incidence of hospital-acquired MRSA bacteraemia in acute general hospitals should focus on improving infection control procedures for the insertion and, most importantly, care of central lines and urinary catheters.
INTRODUCTION
Methicillin-resistant Staphylococcus aureus (MRSA) bacteraemia is one of the most common serious infectious complications of hospitalization. Best estimates suggest that each year there are at least 300 000 cases of hospital-acquired infection in England causing around 5000 deaths [1]. The United Kingdom has the highest reported incidence of hospital-acquired MRSA bacteraemia in Europe [2].
The proportion of S. aureus bacteraemia isolates that are resistant to methicillin in England and Wales increased from <2% in 1990 [3] to 42% in 2002 [4] and the number of cases of MRSA bacteraemia are continuing to rise year on year in England [5] and Wales [6]. Surveillance of all hospital-acquired bacteraemia in English non-teaching hospitals has shown that 22% of cases had a central line, 11% a urinary catheter, and 8% a peripheral line present at the time of, or within 2 days before, the onset of bacteraemia [7]. However, in the absence of any comparison group these data are difficult to interpret. No case-control studies investigating risk factors for hospital-acquired MRSA bacteraemia in the United Kingdom have been published and only a limited number of controlled studies have been undertaken elsewhere.
Routine surveillance in Wales revealed that in one district general hospital rates of MRSA bacteraemia (0·16 cases/1000 bed days) in 2001–2003 were significantly higher than elsewhere in Wales (0·09/1000 bed days) although similar to the mean rate in acute general hospitals in England (0·15/1000 bed days) [8]. We report the findings of a case-control study undertaken, at the request of the hospital's management, to identify risk factors for MRSA bacteraemia.
METHODS
Participants
The study was carried out in an acute district general hospital in Wales serving a predominantly rural catchment area, with an average of 400 beds occupied daily during the period of study. Cases of hospital-acquired MRSA bacteraemia were defined as patients aged ⩾16 years from whom MRSA was isolated from a blood culture taken on the third or subsequent days after admission. Cases were identified from a list of all patients with a blood culture positive for MRSA between 1 April 2002 and 30 September 2003 obtained from the hospital microbiology laboratory. The controls were identified randomly from the hospital patient administration system. Each admission is assigned a unique reference number, the Provider Spell Number. A list of Provider Spell Numbers for admissions between 1 April 2002 and 30 September 2003 was produced and numbered sequentially. Random numbers were generated using Microsoft Excel and used to select provider spells as potential controls. The first 92 provider spells that fulfilled the control definition, patient aged ⩾16 years who stayed in the hospital for more than 2 days, were selected as controls. The study had a statistical power of 80% to detect an odds ratio (OR) of ⩾3 at the 5% significance level, assuming that 30% of controls were exposed to the risk factor under investigation.
Data retrieval
A structured questionnaire was used to collect baseline data (sex, date of birth, date of admission, speciality, date of death or discharge, date of first positive blood culture if appropriate), data on risk factors present on admission (date of most recent previous admission, and the presence of skin ulcers, wounds, bed sores, dermatitis, surgical site infection, in-dwelling urinary or venous catheter, or history of intravenous drug use), and risk factors occurring during admission (insertion of peripheral intravenous line, central line, or urinary catheter, antibiotic treatment, number of contacts with health-care workers and number of inter-ward transfers). Data on risk factors occurring during the hospital stay were recorded for patients with MRSA bacteraemia if they occurred at any time between admission and the sample date of the first MRSA-positive blood culture and for controls if they occurred at any time between admission and discharge or death. Data were retrieved from the hospital patient episode database and from patient notes, including drug charts and the medical, nursing and surgical operation records. Patient notes were scrutinized for information on clinical history, invasive procedures, staff contacts and antibiotic treatment.
Data analysis
Data from completed questionnaires were read into a Microsoft Access database using Eyes & Hands Forms 5 optical character recognition software (ReadSoft AB, Helsingborg, Sweden), and analysed using stata 8.0 (Stata Corporation, College Station, TX, USA) and Epi-Info version 6 (CDC, Atlanta, GA, USA) software. Analysis was limited to cases and controls whose full patient records were available. Time at risk was defined as the time between admission and date of first blood sample positive for MRSA for cases, and time between admission and death or discharge for controls.
The Student's unpaired t test was used to compare normally distributed continuous variables. OR with 95% confidence intervals (CIs) or χ2 were used to compare dichotomous variables. In order to control for the confounding effect of each variable we carried out a logistic regression analysis. The multiple logistic regression analysis included all variables with an OR >2 in the univariate analysis. Time at risk and contact with health-care workers were included as continuous variables in the logistic regression analysis. The population attributable fraction was calculated for the significant variables in the logistic regression model.
RESULTS
Full patient records were available for 42 (91%) of the 46 cases and 90 (98%) of the 92 controls.
Univariate analysis
The mean age of patients was 69 years (median 73, range 35–94 years) in cases compared to a mean of 65 years (median 68, range 19–101 years) in controls (P=0·90). Fifty-seven per cent of cases and 51% of controls were male (P=0·48). Twenty cases (48%) and 40 controls were (45%) admitted to surgical specialities (P=0·77). Five cases but no control had been admitted to the intensive care unit.
Cases were exposed to the hospital setting for a longer period than controls; the mean time at risk for cases was 29 days (median 16, range 3–230) compared to a mean of 12 days (median 7, range 3–154) for controls (P=0·0007). The mean number of contacts with health-care workers recorded in patient notes was significantly higher in cases, 71, than controls, 35 (P<0·0001). The significant risk factors in the univariate analysis were: insertion of a central line, insertion of a urinary catheter, surgical site infection, bed sore, blood transfusion, being at risk for more than 7 days, more than 25 contacts with health-care workers, antibiotic use and peripheral line insertion (Table 1). Sixty-two per cent (26/42) of cases of MRSA bacteraemia had a central line and/or urinary catheter inserted during the admission. Central lines were in situ prior to the onset of bacteraemia for a mean of 18 days (median 16, range 1–69) and urinary catheters were in situ for a mean of 18 days (median 13, range 1–86).
Table 1.
Risk factors for MRSA bacteraemia
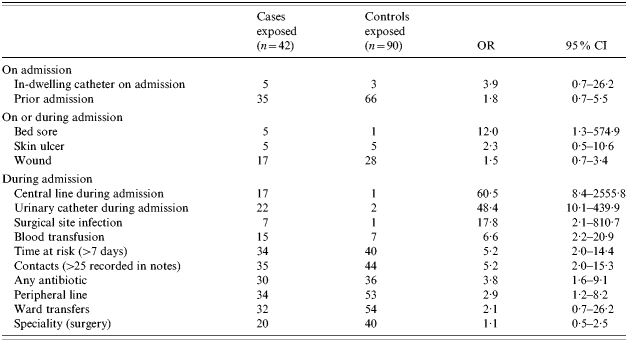
OR, Odds ratio; CI, confidence interval.
Multivariate analysis
Variables included in the logistic regression model were: central line, urinary catheter, surgical site infection, bed sore, blood transfusion, being at risk >7 days, antibiotic use and peripheral line. After adjusting for all factors, having a central line [adjusted OR (aOR) 35·3, 95% CI 3·8–325·5] or a urinary catheter (aOR 37·1, 95% CI 7·1–193·2) inserted during the stay, and suffering from a surgical site infection (aOR 4·3, 95% CI 1·2–14·6) remained independent risk factors for hospital-acquired MRSA bacteraemia. The adjusted population attributable fraction showed that 51% of hospital-acquired MRSA bacteraemia cases in this non-teaching hospital were attributable to a urinary catheter insertion, 39% to a central line and 16% to a surgical site infection (Table 2).
Table 2.
Final model, logistic regression of risk factors for MRSA bacteraemia
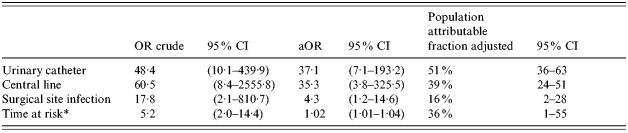
OR, Odds ratio; CI, confidence interval; aOR, adjusted odds ratio.
As a continuous variable for the aOR.
DISCUSSION
Despite the widespread public, professional and political interest in MRSA bacteraemia in UK hospitals no controlled studies specifically investigating risk factors for MRSA bacteraemia have been undertaken in this setting. The only published case-control study in a UK hospital primarily investigated MRSA colonization [9]. None of the published controlled studies from elsewhere in the world investigate risk factors for hospital-acquired MRSA bacteraemia using non-bacteraemic patients as controls. These international studies include investigations of cases S. aureus bacteraemia and non-bacteraemic controls [10], cases of MRSA bacteraemia diagnosed during the first 24 h of admission and non-bacteraemic controls [11], cases of MRSA bacteraemia and patients with methicillin-sensitive S. aureus (MSSA) bacteraemia as controls [12–15], MRSA infection [16, 17], and MRSA colonization [18–25]. Our study identified risk factors for MRSA bacteraemia and provides an estimate of the contribution of these risk factors to the burden of disease in UK hospitals. This should enable interventions to be targeted to areas of care with the greatest potential impact for reducing infection.
To identify risk factors that could potentially be modified in the hospital, cases were restricted to patients from whom MRSA was isolated from a blood sample taken on the third or subsequent day after admission. Samples taken >48 h after admission have previously been used to define nosocomial bacteraemia [16, 26]. It was not possible to use time measured in hours in this study because the time that blood cultures were taken was not routinely recorded. However, the two definitions are broadly comparable and unlikely to result in any important misclassification. We randomly selected the control group from all patients >16 years who had stayed in hospital for more than 2 days.
Pre-admission risk factors
The age and sex of cases and controls in our study was similar. Although population-based studies conducted in Wales have identified the highest incidence of invasive MRSA to be in men aged ⩾75 years [27], age has not been identified as a significant risk factor in other controlled studies [9–11 9–11, 14, 15], and no consistent association with sex has been found with increased risk in males [11, 13], females [15], and no significant differences have been reported [9, 10, 14]. Although previous hospital admission is a risk factor for MRSA colonization at the time of admission [22], our study found no association between previous admission and hospital-acquired MRSA bacteraemia.
Risk factors in hospital
In most instances the development of MRSA bacteraemia is probably a two-stage process with acquisition of the organism and colonization of skin or superficial sites followed, after a variable period, by invasion of the bloodstream. Central or peripheral vascular lines are the most obvious routes for direct invasion into the bloodstream. With one exception [14] other studies that investigated the association between central lines and MRSA bacteraemia also found significant associations that remain in multivariate analysis [10–13]. In our study, central lines were in situ for 18 days on average before the onset of bacteraemia, which suggests that improving the process of line care has a greater potential to reduce bacteraemia than simply looking at the insertion. Although peripheral lines were a significant risk factor in univariate analysis this association disappeared completely in the logistic regression model. Peripheral lines have not been a risk factor in other studies [10] and the apparent association between peripheral lines and MRSA bacteraemia in this study can be explained by confounding.
Time at risk, the time between admission and onset bacteraemia for cases, remained a significant risk factor in the multivariate analysis. This may be a marker of more severe underlying illness in patients that contract MRSA bacteraemia which would tend to result in a longer length of stay in hospital than controls. There was no association between MRSA bacteraemia and the number of recorded contacts with health-care workers in the multivariate analysis. The greater number of contacts recorded for cases can be explained by longer hospital stays. This implies that invasive procedures are more important risk factors for bacteraemia than acquisition of the organism from health-care workers. Most hospital-acquired S. aureus bacteraemias are assumed to originate from organisms that have already colonized the patients for some time [28]. In one study an identical organism was isolated from nasal cultures obtained immediately after the blood isolate in 82% of patients with S. aureus bacteraemia [29]. In another study reported by the latter group of a cohort of patients colonized with S. aureus, the isolate was indistinguishable in 86% of patients who subsequently developed S. aureus bacteraemia [29]. In a third study which followed up a large cohort of patients who were screened on admission to hospital found that in 40% of the patients who developed S. aureus bacteraemia in the subsequent 120 days the isolate was indistinguishable from one that they carried at the time of admission [30].
There was a strong association between MRSA bacteraemia and the insertion of a urinary catheter during the admission. Although patients who have a central line inserted also frequently have a urinary catheter (13 of the 17 patients who had a central line also had a urinary catheter inserted), this does not explain the association. Nine of the 22 cases who had a urinary catheter inserted did not have a central line. It is possible that the observed association between insertion of a urinary catheter during admission and MRSA bacteraemia arises from confounding factors that have not been controlled for in this study.
Interestingly, urinary catheterization has not been linked with MRSA colonization [9], but was associated with MRSA bacteraemia in the only other controlled study to investigate this risk factor [13]. It is plausible that the insertion and/or presence of a urinary catheter are causally associated with hospital-acquired MRSA bacteraemia. Surveillance of device related hospital-acquired bacteraemia suggests that 11% of all bacteraemia and 5% of MRSA bacteraemia is related to urinary catheter-associated infection in non-teaching hospitals [7]. The adjusted population attributable fraction of 51% found in our study suggests that urinary catheters are a more important risk factor for hospital-acquired MRSA bacteraemia than has been previously recognized. Further studies are required to confirm this finding.
It is possible that the risk factors identified in this study may be specific to this hospital and not reflect general risk factors for the acquisition of MRSA bacteraemia. Mandatory surveillance of MRSA bacteraemia, based on identical case definitions and case-finding methodology, was introduced in both England and Wales in 2001 [31]. This study was carried out in an acute general hospital with an incidence of MRSA bacteraemia that is similar to the average rate in England and Wales. We suggest that intervention to reduce MRSA bacteraemia in similar settings should focus on the insertion and care of central lines and urinary catheters. Reducing the extent of use may be impractical since these devices are used in critically ill patients for good reasons and their short-term benefits will usually outweigh the risks. However, as bacteraemia tended to occur after the devices had been in situ for long periods of time continuous review of the indication for their use and removal at the earliest opportunity is desirable. Similarly, concentrating attention on the ongoing care of these devices as well as their insertion may have the greatest potential impact.
In conclusion there have been relatively few case-control studies of MRSA bacteraemia reported in the literature and none have investigated hospital-acquired MRSA bacteraemia using non-bacteraemic patients as controls. Measures to reduce the incidence of hospital-acquired MRSA bacteraemia in acute general hospitals should focus on improving infection control procedures for the insertion and long-term care of central lines and urinary catheters as well as targeting hygiene measures generally.
ACKNOWLEDGEMENTS
We thank the staff of North West Wales Trust's clinical governance, infection control, information management, and medical records departments for their assistance; and administrative and clerical staff at CDSC for their support on data entry. This investigation was undertaken as a clinical review at the request of North West Wales NHS Trust to determine, as a matter of urgency, preventable factors underlying MRSA bacteraemia.
DECLARATION OF INTEREST
None.
REFERENCES
- 1.National Audit Office London, UK: 2004. . Improving patient care by reducing the risk of hospital acquired infection: A progress report. Report by the Comptroller and Auditor General – HC 876 Session 2003–2004. : National Audit Office, [Google Scholar]
- 2.European Antimicrobial Resistance Surveillance System Bilthoven, Netherlands: 2003. . Annual Report 2002. : National Institute of Public Health and the Environment, [Google Scholar]
- 3.Reacher MH et al. Bacteraemia and antibiotic resistance of its pathogens reported in England and Wales between 1990 and 1998: trend analysis. British Medical Journal. 2000;320:213–216. doi: 10.1136/bmj.320.7229.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Public Health Laboratory Service Staphylococcus aureus bacteraemia: England, Wales and Northern Ireland, January to December 2002. Communicable Disease Report CDR Weekly (serial online) 2003 (cited 23 July 2004); 13: Bacteraemia.
- 5.Health Protection Agency . The third year of regional and national analyses of the Department of Health's mandatory MRSA surveillance scheme in England: April 2001–March 2004. Communicable Disease Report CDR Weekly (serial online) 2004 (cited 23 July 2004); 14: Bacteraemia.
- 6.National Public Health Service for Wales Staphylococcus aureus Bacteraemia Surveillance 11th Report. Cardiff, UK: NPHS Communicable Disease Surveillance Centre, 2004 [Google Scholar]
- 7.Coello R et al. Device-related sources of bacteraemia in English hospitals – opportunities for the prevention of hospital-acquired bacteraemia. Journal Hospital Infection. 2003;53:46–57. doi: 10.1053/jhin.2002.1349. [DOI] [PubMed] [Google Scholar]
- 8.National Public Health Service for Wales Staphylococcus aureus Bacteraemia Surveillance 10th Report. Cardiff, UK: NPHS Communicable Disease Surveillance Centre, 2003 [Google Scholar]
- 9.Crowcroft N et al. Methicillin-resistant Staphylococcus aureus: investigation of a hospital outbreak using a case control study. Journal of Hospital Infection. 1996;34:301–309. doi: 10.1016/s0195-6701(96)90110-3. [DOI] [PubMed] [Google Scholar]
- 10.Jensen AG et al. Risk factors for hospital-acquired Staphylococus aureus bacteraemia. Archives of Internal Medicine. 1999;159:1437–1443. doi: 10.1001/archinte.159.13.1437. [DOI] [PubMed] [Google Scholar]
- 11.Tacconelli E et al. Methicillin-resistant Staphylococcus aureus bacteraemia diagnosed at hospital admission: distinguishing between community-acquired versus healthcare-associated strains. Journal of Antimicrobial Chemotherapy. 2004;53:474–479. doi: 10.1093/jac/dkh107. [DOI] [PubMed] [Google Scholar]
- 12.Pujol M et al. Risk factors for nosocomial bacteraemia due to methicillin resistant Staphylococcus aureus. European Journal of Clinical Microbiology and Infectious Diseases. 1994;13:96–102. doi: 10.1007/BF02026134. [DOI] [PubMed] [Google Scholar]
- 13.Selvey LA, Whitby M, Johnson B. Nosocomial methicillin-resistant Staphylococcus aureus bacteremia: is it any worse than nosocomial methicillin-sensitive Staphylococcus aureus bacteremia? Infection Control and Hospital Epidemiology. 2000;21:645–648. doi: 10.1086/501707. [DOI] [PubMed] [Google Scholar]
- 14.Tumbarello M et al. Risk factors and predictors of mortality of methicillin-resistant Staphylococcus aureus bacteraemia in HIV-infected patients. Journal of Antimicrobial Chemotherapy. 2002;50:375–382. doi: 10.1093/jac/dkf126. [DOI] [PubMed] [Google Scholar]
- 15.Cordova SP et al. Methicillin-resistant Staphylococcus aureus bacteraemia in Western Australian teaching hospitals, 1997–1999: risk factors, outcomes and implications for management. Journal of Hospital Infection. 2004;56:22–28. doi: 10.1016/j.jhin.2003.10.009. [DOI] [PubMed] [Google Scholar]
- 16.Graffunder EM, Venezia RA. Risk factors associated with nosocomial methicillin-resistant Staphylococcus aureus (MRSA) infection including previous use of antimicrobials. Journal of Antimicrobial Chemotherapy. 2002;49:999–1005. doi: 10.1093/jac/dkf009. [DOI] [PubMed] [Google Scholar]
- 17.Washio M et al. Risk factors for methicillin-resistant Staphylococcus aureus (MRSA) infection in a Japanese geriatric hospital. Public Health. 1997;111:187–190. doi: 10.1016/S0033-3506(97)00581-7. [DOI] [PubMed] [Google Scholar]
- 18.Dziekan G et al. Methicillin-resistant Staphylococcus aureus in a teaching hospital: investigation of nosocomial transmission using a matched case-control study. Journal of Hospital Infection. 2000;46:263–270. doi: 10.1053/jhin.2000.0846. [DOI] [PubMed] [Google Scholar]
- 19.Scudeller L et al. MRSA carriage: the relationship between community and healthcare setting. A study in an Italian hospital. Journal of Hospital Infection. 2000;46:222–229. doi: 10.1053/jhin.2000.0806. [DOI] [PubMed] [Google Scholar]
- 20.Asensio A et al. Colonization and infection with methicillin-resistant Staphylococcus aureus: associated factors and eradication. Infection Control and Hospital Epidemiology. 1996;17:20–28. doi: 10.1086/647184. [DOI] [PubMed] [Google Scholar]
- 21.Corea E, de Silva T, Perera J. Methicillin-resistant Staphylococcus aureus: prevalence, incidence and risk factors associated with colonization in Sri Lanka. Journal of Hospital Infection. 2003;55:145–148. doi: 10.1016/s0195-6701(03)00256-1. [DOI] [PubMed] [Google Scholar]
- 22.Jernigan JA et al. Prevalence of and risk factors for colonisation with methicillin resistant Staphylococcus aureus at the time of hospital admission. Infection Control and Hospital Epidemiology. 2003;24:409–414. doi: 10.1086/502230. [DOI] [PubMed] [Google Scholar]
- 23.Jernigan JA et al. Prevalence of and risk factors for colonization with methicillin-resistant Staphylococcus aureus in an outpatient clinic population. Infection Control and Hospital Epidemiology. 2003;24:445–450. doi: 10.1086/502223. [DOI] [PubMed] [Google Scholar]
- 24.Young LS, Perdreau-Remington F, Winston LG. Clinical, epidemiologic, and molecular evaluation of a clonal outbreak of methicillin-resistant Staphylococcus aureus infection. Clinical Infectious Disease. 2004;38:1075–1083. doi: 10.1086/382361. [DOI] [PubMed] [Google Scholar]
- 25.Troillet N et al. Carriage of methicillin-resistant Staphylococcus aureus at hospital admission. Infection Control and Hospital Epidemiology. 1998;19:181–185. doi: 10.1086/647791. [DOI] [PubMed] [Google Scholar]
- 26.Laupland KB et al. Population based study of the epidemiology of and the risk factors for invasive Staphylococcus aureus infections. Journal of Infectious Diseases. 2003;187:1452–1459. doi: 10.1086/374621. [DOI] [PubMed] [Google Scholar]
- 27.Morgan M et al. The population impact of MRSA in a country: the national survey of MRSA in Wales, 1997. Journal of Hospital Infection. 2000;44:227–239. doi: 10.1053/jhin.1999.0695. [DOI] [PubMed] [Google Scholar]
- 28.Vaudaux P, Schrenzel J. Nosocomial bacteraemia caused by Staphylococcus aureus. Lancet. 2004;364:644–645. doi: 10.1016/S0140-6736(04)16909-2. [DOI] [PubMed] [Google Scholar]
- 29.von Eiff C et al. Nasal carriage as a source of Staphylococcus aureus bacteraemia. New England Journal of Medicine. 2001;344:11–16. doi: 10.1056/NEJM200101043440102. [DOI] [PubMed] [Google Scholar]
- 30.Wertheim HF et al. Risk and outcome of nosocomial Staphylococcus aureus bacteraemia in nasal carriers versus non-carriers. Lancet. 2004;364:703–705. doi: 10.1016/S0140-6736(04)16897-9. [DOI] [PubMed] [Google Scholar]
- 31.Johnson AP, Pearson A, Duckworth G. Surveillance and epidemiology of MRSA bacteraemia in the UK. Journal of Antimicrobial Chemotherapy. 2005;56:455–462. doi: 10.1093/jac/dki266. [DOI] [PubMed] [Google Scholar]


