SUMMARY
Leptospirosis is one of the most commonly encountered zoonoses in both Australia and the rest of the world. The incidence of leptospirosis in Queensland over the 7-year study period (1998–2004) was 3·1/100 000 population. Enhanced surveillance questionnaires were used to collect patient data and facilitate an epidemiological investigation of leptospirosis in Queensland. Farming occupations comprised the majority of occupational exposure cases, however, recreational exposure accounted for 18% of the 883 cases. Rainfall and the presence of animal hosts had the most influence on the incidence of leptospirosis. Several trends in serovar numbers over this period are noted, in particular the emergence of L. borgpetersenii serovar Arborea, which accounted for 22% of all leptospirosis cases in Australia and 68% of South-East Queensland cases in 2004. Assessment of epidemiological trends in leptospirosis is important to obtain directed public health intervention and outcomes in the reduction of leptospirosis cases.
INTRODUCTION
Leptospirosis is the disease caused by the genus Leptospira. Leptospira are motile spirochaetes of which there are 16 genomospecies as delineated by DNA–DNA hybridization [1]. The genomospecies are divided into pathogenic and non-pathogenic species. Pathogenic species found in Australia include L. interrogans, L. santarosai, L. kirschneri and L. borgpetersenii. In addition to the DNA techniques, a serologically based taxonomy system using cellular antigens is used to divide the Leptospira genomospecies into serovars. There have been over 200 serovars of Leptospira identified [2].
Transmission to humans occurs through penetration of the organism into the blood stream via cuts, skin abrasions or mucus membranes. Urine excretion of the organism by carrier animals, in particular rodents, is the primary environmental source of infections. Workplace infections may also occur through exposure to contaminated body fluids and tissues of animals being slaughtered or handled. The organism will survive in the environment provided that it is moist, warm or is of a favourable pH. In humans, the disease is manifest with symptoms similar to that of other diseases such as dengue, rickettsia, malaria and hepatitis. Specific symptoms of leptospirosis may include chills, malaise, headaches and abdominal pain and severe cases may involve acute renal failure (ARF), jaundice and pulmonary haemorrhage [1, 3].
Acute leptospirosis is diagnosed by the inoculation of blood, urine or CSF cultures from the patient into Ellinghausen–McCullough–Johnson–Harris (EMJH) media and the isolation of the organism. Other diagnostic methods include Leptospira-specific IgM antibody enzyme-linked immunosorbent assays (ELISA) [4] and the amplification of Leptospira-specific DNA using the polymerase chain reaction (PCR) [5, 6]. Seroconversion to Leptospira-specific antibodies occurs generally within 2–3 weeks of infection and diagnosis can then be confirmed using the microscopic agglutination test (MAT). The MAT involves the reaction of total serum antibodies against a panel of antigens in the form of live or attenuated cultures and is examined for agglutination under dark-field microscopy [3]. The MAT is a serovar-specific reaction, with each serogroup represented in the test panel by the serovar(s) most prevalent in that region. Whilst the MAT is a simple method, the end-point interpretation can be subjective.
Leptospirosis is considered an emerging zoonotic disease of global importance and is found in both urban and rural areas of affected countries [1]. In 1999, the International Leptospirosis Society (ILS) published a limited snapshot of the worldwide distribution of the disease [7]. The disease was most prevalent in countries with large rural and developing areas such as China, Brazil, India, Romania and Russia. More deaths occurred in these countries, where the limited availability of medical attention is directly linked to the developing nature of the country [7].
Leptospirosis was first reported in Australia in 1933, when 40 cases with similar aetiology were notified over a period of 5 months from the township of Ingham in the state of Queensland. Diagnosis was made through histological examination of autopsy material. Leptospirosis was not reported until the early 1950s in the seven other states and territories of Australia [8–10]. Several Leptospira serovars were first isolated in Australia. These include; L. interrogans serovars (sv.) Australis, Zanoni, Kremastos, Robinsoni, Broomi, Pomona, Szwajizak; L. kirschneri sv. Valbuzzi; L. weilli sv. Celledoni and L. meyeri sv. Perameles. All of these serovars were isolated from human patients with the exception of L. meyeri sv. Perameles which was isolated from a bandicoot (Perameles nasuta) [11].
The aim of this study was to establish the current trends in the epidemiology of leptospirosis especially the emergence of L. borgpetersenii sv. Arborea for the state of Queensland based upon extended surveillance undertaken by the WHO/FAO/OIE Collaborating Centre for Reference & Research on Leptospirosis over the period 1998–2004.
METHODS
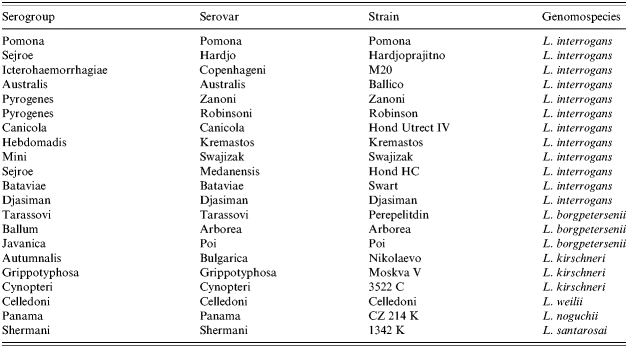
Notification criteria for leptospirosis in Australia are a positive IgM ELISA supported by a single MAT titre of ⩾400, or a fourfold rise or fall in MAT titres over paired specimens, or isolation of the organism. All serum specimens in this study returned a positive result by screening tests such as the Leptospira-specific IgM ELISA at secondary-level laboratories and were positive by MAT when tested at the WHO/FAO/OIE Collaborating Centre for Reference & Research on Leptospirosis (reference laboratory). All other notifications were positive isolations. The MAT panel used by the reference laboratory consisted of 21 live Leptospira serovars from 19 serogroups representative of those found in the Western Pacific Region (Table 1). Leptospires were isolated by inoculation of 3–5 drops of whole blood from the patient into EMJH medium and incubated at 30°C for up to 6 weeks. The infecting serovar from positive isolations was identified by the cross-agglutinin absorption test (CAAT). Positive MAT and isolations are notified to the Queensland Health Communicable Diseases Centre and to the Surveillance Section, Bio-security and Disease Control Branch, Commonwealth Department of Health and Ageing, Canberra. Extended surveillance forms were completed by the requesting doctor or a public health nurse and returned to the reference laboratory. Basic demographic data was sourced from Auslab, the Queensland Health reporting and laboratory information management system. Information from extended surveillance forms entered into a Microsoft Access database provided data on symptoms, recreational activities, animal contacts, occupational data and hospitalization. Microsoft Excel was used to conduct statistical analysis of surveillance data. Rodent trapping was conducted over a period of 7 years in various regions of Queensland to determine which Leptospira serovars were present in the rodent populations. Kidneys, tissue and urine samples were cultured in EMJH semi-solid agar for up to 6 weeks at 30°C. Any isolates recovered were identified by CAAT.
Table 1.
Leptospira serovars used in the MAT panel at the WHO/FAO/OIE Collaborating Centre for Reference and Research on Leptospirosis
RESULTS
Epidemiology of leptospirosis in Queensland
The total number of leptospirosis notifications reported to the Surveillance Section, Bio-security and Disease Control Branch, Commonwealth Department of Health and Ageing, Canberra, Australia from 1934 until 2004 was 7507 cases. Division of these notifications on a state-by-state basis shows that over the 70-year period New South Wales had 1288 cases (17·2%), Victoria had 1225 cases (16·3%), South Australia had 236 cases (3·2%), Western Australia had 226 cases (3·0%), Tasmania had 147 cases (2·0%), the Northern Territory had 34 cases (0·5%), the Australian Capital Territory had 26 cases (0·3%) and Queensland had 4325 cases (57·6%) [12].
There were a total of 886 leptospirosis notifications for Queensland over the 7-year study period with incidence rate averaging 3·2 cases/100 000 population. The year with the highest incidence was 1999 with 6·2 cases/100 000 population and the lowest year was 1997 with 1·6 cases/100 000 population. The national average for 1998–2004 was 1·1 cases/100 000 population [12].
The disease was more prevalent in males than in females with males accounting for 92·6% (n=883) of cases. The ratio of male to female cases was 25:2. The median age of persons affected was 34 years, whilst the age group most affected was 21–40 years with 49·0% of cases, followed by the 41–60 years age group (29·6%). The range of ages affected was from 70 to 80 years.
Hospitalization was reported in 51·8% of cases (n=883). The median stay in hospital was 3 days and the longest period of hospitalization was 85 days. L. interrogans svs. Australis, Zanoni and L. borgpetersenii sv. Arborea infections resulted in a slightly longer than average hospitalization period of ∼5 days. There have been no proven fatalities from leptospirosis during this period.
The major symptoms reported in the 883 cases were; headaches (55%), severe fever (52%), myalgia (48%), chills (48%), sweats (48%), arthralgia (38%), nausea (35%), vomiting (33%), back pain (27%), renal impairment (13%) and liver impairment (10%). Two importantly recognized symptoms of leptospirosis are pulmonary haemorrhage and ARF. Pulmonary haemorrhage occurred in 31 cases. The serovars involved with the majority of the pulmonary haemorrhage cases were L. interrogans sv. Australis (32·3%) and L. interrogans sv. Zanoni (48·4%). Renal failure was noted in 12 cases, and in parallel with pulmonary haemorrhage, the major infecting serovars causing renal failure were L. interrogans sv. Australis (41·7%) and L. interrogans sv. Zanoni (33·3%).
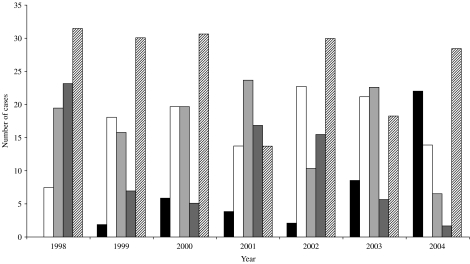
Over the 7-year period, 21 serovars of Leptospira have been detected in Queensland by MAT and/or culture. The majority of the 883 cases were caused by L. interrogans sv. Zanoni (26·7%), L. interrogans sv. Australis (16·5%) and L. borgpetersenii sv. Hardjo (16·6%). Other serovars prominent during this period included L. interrogans sv. Pomona (10·2%), L. interrogans sv. Kremastos (4·0%), L. interrogans sv. Szwajizak (5·0%), L. borgpetersenii sv. Tarassovi (4·6%) and L. borgpetersenii sv. Arborea (5·9%). Several trends can be noted in the infecting serovars detected over the period with the notifications of L. interrogans sv. Australis and L. interrogans sv. Zanoni remaining stable, making up approximately 20% and 30% of cases per year respectively. A slightly downward trend is seen in the number of L. interrogans sv. Pomona and L. borgpetersenii sv. Hardjo notifications, with an upward trend noted in the number of L. borgpetersenii sv. Arborea cases (Fig. 1).
Fig. 1.
Trends in the five major serovars detected in Queensland from 1998 to 2004. ■, Arborea; □, Australis;
 , Hardjo;
, Hardjo;
 , Pomona;
, Pomona;
 , Zanoni.
, Zanoni.
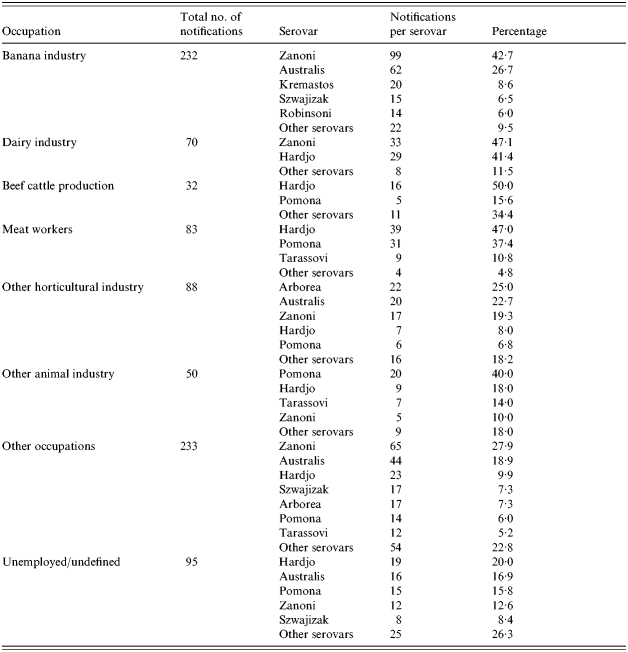
When occupational data was examined, occupations associated with agriculture accounted for 62·9% (n=883) of cases across the 7-year period, 26·3% were non-agricultural based and the remainder were unemployed or undefined (10·8%). Banana industry workers accounted for 26·3% of total Queensland cases (n=883), whilst other significant industries included dairy farming (7·9%), beef cattle production (6·1%) and meat processing (9·4%). Cane farming accounted for only 1·8% of total cases. Several serovars had a strong association with certain industries; L. interrogans sv. Australis and L. interrogans sv. Zanoni with the banana industry, L. interrogans sv. Zanoni and L. borgpetersenii sv. Hardjo with dairy farming and L. borgpetersenii sv. Hardjo and L. interrogans sv. Pomona with the meat processing industry (Table 2).
Table 2.
Occupations and major infecting serovars of Leptospira found in Queensland, 1998–2004
Leptospirosis acquired by recreational exposure accounted for 157 of the 883 (17·8%) cases across the 7 years. The recreational activities most commonly associated with acquiring leptospirosis included bushwalking, swimming, camping and shooting/hunting.
As leptospirosis is a zoonotic disease, determining the possible animal vectors is important in breaking the disease cycle. Animals such as rats, dogs, mice, cattle, feral/domestic pigs and cats were frequently reported. However, as some serovars are indigenous to Australia, native animals need to be recognized as a source of infection; contact with kangaroos, wallabies, native rats, possums and bandicoots was reported in 153 of the 883 cases (17·3%).
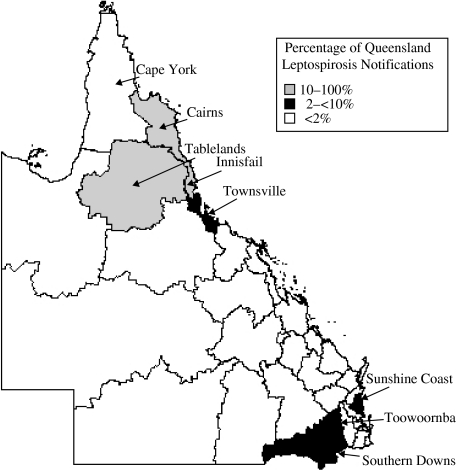
Queensland is divided into 30 health districts with several of these being recognized as areas of endemic leptospirosis [9]. The majority of Queensland's leptospirosis notifications (n=886) occur in the Cairns (12·3%), Tablelands (14·9%) and Innisfail districts (38·3%). The remaining health districts each account for <2% of all Queensland cases with the exception of the Southern Downs (4·3%), Toowoomba (3·6%), Townsville (3·2%) and Sunshine Coast (2·8%) districts (Fig. 2). In the Cairns district, the major serovars detected from the 109 cases were L. interrogans sv. Australis (28·4%), L. interrogans sv. Canicola (9·2%), L. borgpetersenii sv. Hardjo (11·0%) and L. interrogans sv. Zanoni (22·0%). In 132 cases from the Tablelands district, the majority of cases were L. interrogans sv. Hardjo (31·1%) and L. interrogans sv. Zanoni (55·3%). In the Innisfail district, the dominant serovars from 338 cases were L. interrogans sv. Australis (25·7%) and L. interrogans sv. Zanoni (37·9%), and to a lesser extent, L. interrogans sv. Robinsoni (7·7%), L. interrogans sv. Szwajizak (8·6%) and L. interrogans sv. Kremastos (8·6%). In more temperate districts of Queensland such as the Southern Downs and Sunshine Coast there was less variation in identified serovars. The major serovars detected in the 37 cases from the Southern downs district were L. borgpetersenii sv. Arborea (16·2%), L. borgpetersenii sv. Hardjo (29·7%) and L. interrogans sv. Pomona (40·5%). Similarly in the Sunshine Coast, L. interrogans sv. Pomona (12·0%), L. borgpetersenii sv. Hardjo (28·0%) and L. borgpetersenii sv. Arborea (48·0%) made up the majority of the 25 cases over the 7-year period.
Fig. 2.
The distribution of leptospirosis cases over 30 health districts in the state of Queensland.
Typically the majority of leptospirosis cases are seasonally distributed throughout the year with 50·6% of cases being detected during the autumn months of March, April and May (n=883). The remainder of cases occurs throughout the summer and winter months with 21·3% and 18·7% respectively whilst the lowest number of cases occurs in the spring period (Fig. 3).
Fig. 3.
The effect of rainfall on the incidence of leptospirosis. This data is based on the average rainfall data (□) [13] and average leptospirosis notifications (–◆–) from the Innisfail Health District over the 7-year study period.
The total mean rainfall received in Innisfail, the area with the highest number of notifications for leptospirosis, is 3559 mm compared to the Redcliffe/Caboolture district with 1094 mm and North Burnett district with 766 mm [13], areas which have the lowest number of notifications for the 7-year period. A comparison of average rainfall and the average incidence of leptospirosis in the Innisfail District is shown in Figure 3. Rainfall increases steadily from December and peaks in March coinciding with the monsoon or ‘wet’ season and then decreases to <200 mm per month from June to November. Leptospirosis cases in the Innisfail region increase from January, with the highest case numbers from March to May and a sharp decrease from May to June. There is little leptospirosis activity seen from August to December during the ‘dry’ season. The temperature in this region is quite stable and averages 27°C throughout summer and 21°C throughout winter [13].
Emergence of L. borgpetersenii sv. Arborea in Queensland
The first notification of human infection with L. borgpetersenii sv. Arborea in Australia occurred in 1998 when a 20-year-old male from Northern New South Wales was diagnosed by MAT with a titre of 1:1600 against the reference strain L. borgpetersenii sv. Arborea. In the same year, the Reference laboratory commenced rodent trapping in South-East Queensland, collecting 178 rodent species. Leptospires were isolated from five rodent kidney cultures and were identified as L. borgpetersenii sv. Arborea by the CAAT. In 2000, further rodent trapping was undertaken in South-East Queensland after several unusual leptospirosis cases, including a case of Arborea, were diagnosed serologically from humans in the region. A further six isolates were recovered from cultures of 15 rodent specimens and identified as L. borgpetersenii sv. Arborea by CAAT. In total, there have been 12 isolations of L. borgpetersenii sv. Arborea from animals in Queensland. All have been isolated from introduced species of rodents and the majority of isolations were from South-East Queensland with the exception of one isolate found in Innisfail, North Queensland.
There have been a total of 66 human cases of L. borgpetersenii sv. Arborea notified in Australia from 1998 to 2004. Of these, six were diagnosed by culture, while the remaining 60 were serologically identified using MAT. However, given that both L. borgpetersenii sv. Ballum and L. borgpetersenii sv. Arborea react similarly in the MAT, the identification of these cases as L. borgpetersenii sv. Arborea is based upon the lack of evidence that L. borgpetersenii sv. Ballum exists in Australia as it has never been isolated. L. borgpetersenii sv. Arborea cases have averaged six per year over the 7-year study period. In 2000, there were 10 notifications for L. borgpetersenii sv. Arborea; this was due to a cluster of five cases from the northern suburbs of Brisbane with all cases employed at the same workplace. The subsequent investigation implicated an infestation of mice in the building as the likely source of the infection. Mice had been reported in previous weeks around the lunch facilities and rodent faeces were found inside the premises. In 2004 L. borgpetersenii sv. Arborea notifications increased to 32, which represented 22% of all Australian leptospirosis notifications (n=146) and constituted 68% of all South-East Queensland leptospirosis notifications (n=36). In contrast from 1998 to 2003, L. borgpetersenii sv. Arborea represented only 3% of the 1078 notifications in Australia; whilst in South-East Queensland L. borgpetersenii sv. Arborea represented 15% of the 160 notifications for that region.
The majority of L. borgpetersenii sv. Arborea cases (92·4%) originate in South-East Queensland and neighbouring Northern New South Wales. The remaining notifications were from outside of this area with three from North Queensland and two from Southern New South Wales.
The predominant occupations for L. borgpetersenii sv. Arborea cases were those in the horticultural/grain industries which accounted for 36·4% of notifications with the majority being small crop and vegetable farmers. A further 19·7% of cases were from those involved in animal-based occupations such as meat processing, graziers and dairy farmers. Occupations not traditionally associated with leptospirosis accounted for 31·8% of cases (n=66) however most had reported some form of contact with either rodents or cattle.
DISCUSSION
The incidence of leptospirosis in Queensland for the 7-year period was approximately three times that of the national average for the same period. The main factors for this appear to be climate, geography, occupational exposure, animal species present in the region and recreational exposure. Figure 2 shows several regions with higher levels of leptospirosis incidence including Cairns, Tablelands and Innisfail districts. These districts have a tropical climate with periods of very high rainfall during the wet season. When compared to regions of similar climate but lower incidence of leptospirosis such as Cape York, the two major differences are higher population densities and the intensity of agricultural activity. South- East Queensland also shows higher levels of leptospirosis in comparison to surrounding areas. South-East Queensland is the most densely populated region of the state as well as having high levels of agricultural activity. The Central West, South-West and North-West areas of Queensland show little leptospirosis activity, as these areas are drier than the coastal strip with a low population density of <0·2 persons/km2 [14]. Drier environments are less likely to support the survival of leptospires outside of a host and combined with a shortage of human hosts, could account for very low leptospirosis incidence (<2 cases/100 000 population).
The effect of rainfall on leptospirosis incidence is illustrated by Figure 3. This figure is typical of an area that experiences distinct ‘wet’ and ‘dry’ seasons such as North Queensland. The majority of rain is experienced during the summer monsoon season with the peak in March. Leptospirosis cases parallel this upward trend from January to May with little activity seen in the second drier half of the year. There is a time difference of about a month between rainfall and leptospirosis case numbers due in part to notification date being based upon the date specimens are received in the laboratory and also due to the 10- to 14-day incubation period of the disease.
Occupationally, workers in the banana industry appear to be most at risk of contracting leptospirosis. This type of work involves a high level of manual labour in a generally moist environment. Workers may not always wear suitable protective clothing due to the high humidity and mud and may also come into contact with native rats in and around banana plants. Of particular interest is the contrast to the sugar-cane industry, which has become a highly mechanized industry and represented only 1·8% of the 883 cases. Prior to mechanization in the 1960s, sugar-cane workers accounted for 26·2% of cases in Queensland [8]. The major cause of leptospirosis in industries such as dairy farming and meat processing is the requisite close contact with livestock and direct exposure to animal urine or infected body tissues. Occupations not traditionally associated with leptospirosis account for nearly one in five cases in this study. These notifications are due mainly to exposure through recreational activities such as bushwalking, hunting, gardening and water-based activities. In the future, recreational exposure may account for a larger percentage of cases as recreational activities and tourism involving direct contact with the environment becomes more popular.
Several trends in serovars have become apparent over the 7-year period. Serovars such as L. interrogans sv. Zanoni and L. interrogans sv. Australis have remained at around the same level in comparison to total cases. These serovars are carried by native animals such as rodents and small marsupials, and human contact with these animals and their environment remains constant. L. interrogans sv. Pomona and L. borgpetersenii sv. Hardjo have displayed a slight downward trend. In Queensland, these serovars are likely to be carried by livestock such as cattle or pigs and a large proportion of these cases are from those involved in livestock-based industries such as dairy farming, meat processing or beef cattle production. The decline in numbers of L. interrogans sv. Pomona and L. borgpetersenii sv. Hardjo cases may be due to number of factors such as increased worker awareness of the disease, the greater use of personal protective equipment, and the use of leptospirosis vaccines within herds.
The emergence of L. borgpetersenii sv. Arborea in Queensland
L. borgpetersenii sv. Arborea is one of the five known members of the Ballum serogroup and was first isolated in Europe in 1944 [11]. The main carriers of L. borgpetersenii sv. Arborea throughout the world is rodents, in particular; Mus domesticus and Rattus rattus [15–17]. Prior to 1998, L. borgpetersenii sv. Arborea had not been isolated from humans in Australia although studies conducted in Victoria in the early 1980s noted serological evidence of exposure to the Ballum serogroup in cattle, horses and native animals [18–20]. The reference laboratory has included members of the Ballum serogroup in the routine MAT panel (Table 1) since 1991 to capture cases acquired overseas. Interestingly there were no serogroup Ballum infections recorded in Queensland from 1991 to 1997. The reasons for the sudden increase in notifications of L. borgpetersenii sv. Arborea throughout the 7-year study period remains unclear. Given that L. borgpetersenii sv. Arborea is linked with the imported rodent population, it could be postulated that the increases are due to the colonization of a pocket of rodents by L. borgpetersenii sv. Arborea, an increase in rodent contact with humans and/or an increase in rodent numbers during that year. Other hypotheses for the increase in L. borgpetersenii sv. Arborea notifications include increased awareness of physicians in South-East Queensland to consider leptospirosis as a differential diagnosis in pyrexia of unknown origin (PUO) cases or that L. borgpetersenii sv. Arborea has existed in Queensland but has not been detected or the serovar has been present in rodent colonies that have not had contact with humans.
It remains to be seen whether this increase in L. borgpetersenii sv. Arborea will become a permanent feature of case numbers in the future or whether it is merely an aberration.
Leptospirosis continues to pose a significant public health problem in Queensland despite 50 years of research, public health investigations and epidemiological review of the disease. Whilst the occupational trends have changed since the 1950s, exposure to contaminated soil/water through agricultural or recreational activities still accounts for the majority of infections. The emergence of L. borgpetersenii sv. Arborea in Queensland is possible evidence of how an imported serovar of Leptospira can be distributed geographically via the resident non-native rodent population and how it can impact on areas that have had little history of leptospirosis. Continued monitoring of the epidemiology of leptospirosis is needed to ascertain whether recreational exposure is increasing or how the emergence of serovars such as L. borgpetersenii sv. Arborea, will impact on non-endemic leptospirosis areas and occupations.
ACKNOWLEDGEMENTS
The authors thank the public health unit and hospital staff for their assistance in the surveillance programme to date and also Mr Michael Krause and Mr Shane Byrne, for their help in the preparation of this manuscript.
DECLARATION OF INTEREST
None.
REFERENCES
- 1.Bharti AR et al. Leptospirosis: a zoonotic disease of global importance. Lancet Infectious Diseases. 2003;3:757–771. doi: 10.1016/s1473-3099(03)00830-2. [DOI] [PubMed] [Google Scholar]
- 2.Faine S, Adler B, Bolin C. Leptospira and leptospirosis. Melbourne, Australia: MediSci; 1999. [Google Scholar]
- 3.Levett PN. Leptospirosis. Clinical Microbiology Reviews. 2001;14:296–326. doi: 10.1128/CMR.14.2.296-326.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Flannery B et al. Evaluation of recombinant Leptospira antigen-based enzyme-linked immunosorbent assays for the serodiagnosis of leptospirosis. Journal of Clinical Microbiology. 2001;39:3303–3310. doi: 10.1128/JCM.39.9.3303-3310.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Levett PN et al. Detection of pathogenic leptospires by real-time quantitative PCR. Journal of Medical Microbiology. 2005;54:45–49. doi: 10.1099/jmm.0.45860-0. [DOI] [PubMed] [Google Scholar]
- 6.Smythe LD et al. A quantitative PCR (TaqMan) assay for pathogenic Leptospira spp. BMC Infectious Diseases. 2002;2:13. doi: 10.1186/1471-2334-2-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.World Health Organization. Leptospirosis worldwide, 1999. Weekly Epidemiological Record. 1999;74:237–242. [Google Scholar]
- 8.Johnson DW. The Australian leptospiroses. Medical Journal of Australia. 1951;2:724–731. doi: 10.5694/j.1326-5377.1950.tb106863.x. [DOI] [PubMed] [Google Scholar]
- 9.Morrisey GC. The occurrence of leptospirosis (Weil's disease) in Australia. Medical Journal of Australia. 1934;2:496–497. [Google Scholar]
- 10.Wellington NAM, Stevenson WJ, Ferris AA. Endemic leptospirosis in Victoria. Medical Journal of Australia. 1951;1:15–18. doi: 10.5694/j.1326-5377.1951.tb70641.x. [DOI] [PubMed] [Google Scholar]
- 11.Kmety E, Dikken H. Classification of the Species Leptospira interrogans and History of its Serovars. 1st edn. Groningen, Netherlands: University Press Groningen; 1993. [Google Scholar]
- 12.Nationally Notifiable Diseases (NNDSS) http://www.health.gov.au/internet/wcms/publishing.nsf/Content/Nationally+notifiable+diseases+%28NNDSS%29-1. http://www.health.gov.au/internet/wcms/publishing.nsf/Content/Nationally+notifiable+diseases+%28NNDSS%29-1 ). Accessed 1 July 2005.
- 13.Bureau of Meteorology. http://www.bom.gov.au/ http://www.bom.gov.au/ ). Accessed 1 July 2005.
- 14.Australian Bureau of Statistics. http://www.abs.gov.au/ http://www.abs.gov.au/ ). Accessed 9 February 2006.
- 15.Everard CO et al. A twelve-year study of leptospirosis on Barbados. European Journal of Epidemiology. 1995;11:311–320. doi: 10.1007/BF01719436. [DOI] [PubMed] [Google Scholar]
- 16.Collares-Pereira M et al. Rodents and Leptospira transmission risk in Terceira island (Azores) European Journal of Epidemiology. 2000;16:1151–1157. doi: 10.1023/a:1010916132497. [DOI] [PubMed] [Google Scholar]
- 17.Matthias MA, Levett PN. Leptospiral carriage by mice and mongooses on the island of Barbados. West Indian Medical Journal. 2002;51:10–13. [PubMed] [Google Scholar]
- 18.Milner AR, Wilks CR, Calvert K. The prevalence of antibodies to members of Leptospira interrogans in cattle. Australian Veterinary Journal. 1980;56:327–330. doi: 10.1111/j.1751-0813.1980.tb05741.x. [DOI] [PubMed] [Google Scholar]
- 19.Milner AR et al. The prevalence of anti-leptospiral agglutinins in sera of wildlife in southeastern Australia. Journal of Wildlife Disease. 1981;17:197–202. doi: 10.7589/0090-3558-17.2.197. [DOI] [PubMed] [Google Scholar]
- 20.Swart KS, Calvert K, Meney C. The prevalence of antibodies to serovars of Leptospira interrogans in horses. Australian Veterinary Journal. 1982;59:25–27. doi: 10.1111/j.1751-0813.1982.tb02707.x. [DOI] [PubMed] [Google Scholar]