SUMMARY
Despite the importance of lower respiratory-tract infection (LRI) in causing hospitalizations in elderly patients (⩾65 years of age) and recent advances in vaccine development, a complete picture of the causative organisms is not available. All hospital discharge diagnoses (ICD-10 code) for LRI in elderly patients in England during 1995–1998 were reviewed. Using known seasonality in potential causative agents of LRI, the contribution of different respiratory pathogens to hospitalizations coded as ‘unspecified LRI’ was estimated by multiple linear regression analysis. Ninety-seven per cent of 551 633 LRI-associated diagnoses had no specific organism recorded. From the statistical model the estimated proportions of admissions attributable to different pathogens were applied to calculate estimated hospitalization rates: 93·9 hospitalizations/10 000 population aged ⩾65 years due to S. pneumoniae, 22·9 to influenza virus, 22·3 to H. influenzae, 17·0 to whooping cough, and 12·8 to respiratory syncytial virus. There is enormous potential to improve health using existing vaccines and those under development.
INTRODUCTION
Despite the importance of lower respiratory-tract infection (LRI) in causing hospital admissions in the elderly, a complete picture of the causative organisms has not been available for a number of reasons. For some organisms, most notably respiratory syncytial virus (RSV) and Bordetella pertussis a role in causing LRI in the elderly has only been recognized relatively recently [1–4]. In general, clinicians do not investigate for virological causes of LRI such as influenza virus or RSV in adults because a specific diagnosis does not necessarily change management of patients in the absence of any treatment. If clinicians do investigate, they still may not obtain a specific diagnosis because significant change occurs with age in some laboratory values [5] or they may face the problem that blood cultures are insensitive methods to diagnose, for example Streptococcus pneumoniae, a major bacterial cause of LRI. Consequently, laboratory-based surveillance grossly under-ascertains morbidity from the causative organisms of LRI in the elderly.
Several factors are increasing the priority of having good estimates of the contribution of each organism to the burden of LRI and of how much can be prevented. The place of new vaccines needs to be defined, including the licensed conjugate pneumococcal vaccine and those in development for infections such as RSV [6]. Changes in existing vaccination programmes such as those implemented in the United Kingdom for influenza in 2000 [7] and consideration of new pneumococcal vaccines would benefit from more comprehensive evaluation.
Increasing antibiotic resistance has led to policies to minimize unnecessary antibiotic use and to promote use of narrow-spectrum antibiotics targeted at specific agents. These require accurate microbiological diagnosis. In addition, anti-viral agents have been developed [8], and new agents are in development. A better understanding of the role of different organisms in causing LRI admissions may help direct rational use of anti-bacterial and anti-viral agents in hospitals.
In the past community-based studies have aimed to produce a comprehensive picture. These have been limited for a number of reasons. Some studies have been biased towards either bacterial or viral causes of LRI and most have identified the aetiological agent in the minority of recruited patients [9, 10]. Although advanced diagnostic methods such as polymerase chain reaction assays have improved diagnostic yield, such studies still have to be large and run for several years to include organisms with epidemic cycles, making them costly and difficult to carry out.
Modelling has the potential to complete the patchy picture given by surveillance and sporadic descriptive studies. In this study we use a simple statistical model to estimate the proportion of unspecified pneumonia, bronchitis and chronic obstructive pulmonary disease (COPD) related hospitalizations in the elderly due to S. pneumoniae, RSV, influenza, B. pertussis and other important infectious causes of respiratory disease.
METHODS
Hospitalization data
The number of all patients hospitalized with a diagnosis of LRI in England was obtained from the hospital episodes statistic (HES) database from April 1995 to March 1998. This database contains the personal, medical and administrative details of all patients admitted to NHS hospitals in England. This represents a population of ∼49 million people. Using personal identifiers such as date of birth and postal code, duplicates were identified and excluded.
Elderly patients (⩾65 years of age) were selected if the diagnosis in any of the seven diagnostic fields in their HES record matched the following International Classification of Diseases, 10th Revision (ICD-10) codes for LRI: pneumonia (J12-18), acute bronchitis (J20), acute bronchiolitis (J21), unspecified acute lower respiratory infection (J22), bronchitis, not specified as acute or chronic (J40), influenza (J10, J11), other chronic obstructive pulmonary disease with acute lower respiratory tract infection/acute exacerbation, unspecified (J44), whooping cough (A37), Legionnaires’ disease (A48), Chlamydia psittaci infection (A70).
Statistical model
Multiple linear regression analysis was applied to estimate the proportion of unspecified LRI hospitalizations to different respiratory pathogens in the elderly. These statistical models were constructed as described previously [11, 12]. The technique uses the observed temporal variation in potential causative pathogens for pneumonia, bronchitis and COPD to estimate the level of under-diagnosis for each of these causes.
The dependent variables in the regression analysis were the weekly number of admissions in patients aged ⩾65 years due to unspecified pneumonia (ICD-10: J12.8/9; J15.8/9; J18), unspecified bronchitis/bronchiolitis (ICD-10: J20.9, J21.9) and unspecified COPD (ICD-10: J44.0/1), respectively. Admissions with a LRI due to whooping cough, Chlamydia, Escherichia coli, Haemophilus influenzae, influenza virus, Klebsiella pneumoniae, Legionella spp., Mycoplasma pneumoniae, parainfluenza virus, rhinovirus, RSV, S. pneumoniae, streptococci other than S. pneumoniae, and Staphylococcus were included as independent variables. Also, a variable named ‘other’ was defined to account for organisms not sufficiently numerous to include separately (coxsackievirus, echovirus, Pseudomonas) and for non-infectious causes of LRI (allergic asthma, non-allergic asthma, mixed asthma). The variable ‘dual infections’ included admissions due to more than one of the above-mentioned causative organisms.
After confirming that the underlying seasonal pattern was the same in all age groups, data from all age groups were used for the independent variables to make the seasonal trends more distinct. Backwards stepwise regression was performed to remove variables that did not contribute to the model using SPSS for Windows, version 10.0 (SPSS Inc., Chicago, IL, USA). In a step-by-step procedure single variables that reduced R2 by the smallest increment were removed from the equation if the resulting decrease was not statistically significant by the F test (significance level of F value <0·05). The procedure was continued until the removal of a variable caused a significant reduction in the overall model fit.
Validity of the final models (modelpneumonia, modelbronchitis and modelCOPD) was confirmed by analysis of residual plots. The analysis was performed using SPSS for Windows, version 10.0 (SPSS Inc.).
Calculating hospitalization rates
The observed hospitalization rates for S. pneumoniae, influenza virus, H. influenzae, whooping cough, RSV, streptococci other than S. pneumoniae, and K. pneumoniae were calculated using denominators derived from the annual resident population estimates based on the projections of the 1991 UK census (Office for National Statistics).
From the statistical model the estimated proportions of admissions attributable to the above-mentioned respiratory pathogens were applied to calculate estimated hospitalization rates for each pathogen.
RESULTS
Hospitalizations in the elderly, HES data
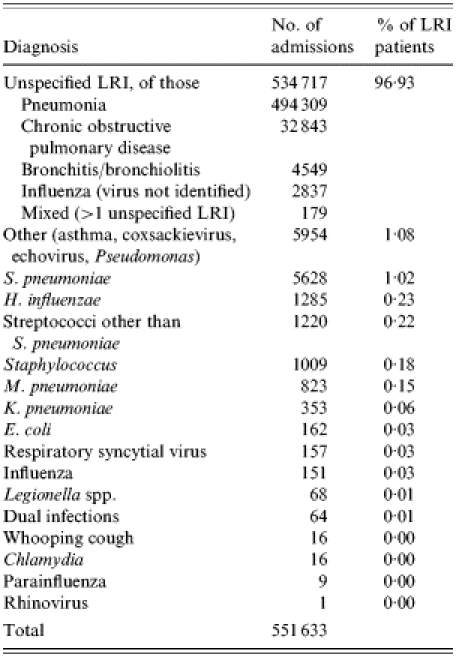
HES records associated with LRI were found for 551 633 patients aged ⩾65 years during the 3-year study period. Table 1 shows the number and diagnosis of LRI-associated hospitalizations. Ninety-seven per cent of these admissions did not have a specific organism recorded in any of the diagnostic fields, and are defined here as ‘LRI due to unspecified organism’. Of these unspecified diagnoses, 92% were diagnosed as pneumonia, 6% as COPD, and <1% were diagnosed as bronchitis/bronchiolitis, and ‘influenza (virus not identified)’ respectively. Of the remaining 16 916 patients with a specific diagnosis for LRI, 33% were diagnosed with S. pneumoniae, 8% with H. influenzae, 7% with streptococci other than S. pneumoniae, 6% with Staphylococci, 5% with M. pneumoniae, and 2% with K. pneumoniae. Less than 1% of the patients were diagnosed with E. coli, RSV, influenza virus, Legionella spp., parainfluenza virus, rhinovirus whooping cough, Chlamydia, or with more than one specific LRI (‘dual infection’) respectively. Thirty-five per cent of the patients belonged to the category ‘other’ (allergic asthma, non-allergic asthma, mixed asthma, coxsackievirus, echovirus, Pseudomonas).
Table 1.
Number and diagnosis of lower respiratory-tract infection (LRI)-associated hospitalizations in the elderly (⩾65 years), England, April 1995 to March 1998
Results of statistical models for unspecified pneumonia, bronchitis, and COPD
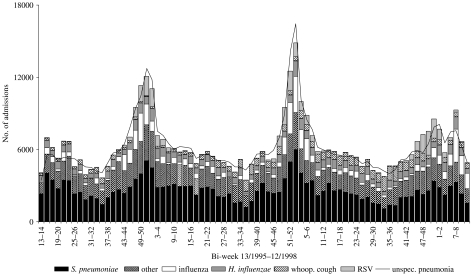
Unspecified LRI-associated hospitalizations in the elderly due to specific respiratory pathogens were estimated using three models: modelpneumonia, modelbronchitis and modelCOPD. Table 2 demonstrates the modelpneumonia for unspecified pneumonia. The variables admission due to whooping cough, H. influenzae, influenza virus, RSV, S. pneumoniae, and ‘other’ remained in the final model. The adjusted R2 of modelpneumonia indicates that 90% of the variation in the weekly number of unspecified cases was explained by the model. Figure 1 demonstrates both the fit of the model and what proportion of hospitalizations are caused by the different organisms at different times. Using this model, it was estimated that 42·1% of unspecified pneumonia could be attributed to S. pneumoniae, 9·8% to influenza virus, 9·1% to H. influenzae, 7·4% to whooping cough, 5·1% to RSV and 26·6% to other causes (Table 3).
Table 2.
Covariates in modelpneumonia
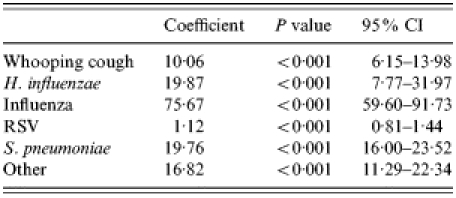
RSV, respiratory syncytial virus; CI, confidence interval.
Fig. 1.
Comparison of observed bi-weekly number of unspecified pneumonia hospitalizations in the elderly (⩾65 years) with estimated number due to S. pneumoniae, influenza. B. pertussis, respiratory syncytial virus (RSV), and other causes based on the final modelpneumonia shown in Table 2.
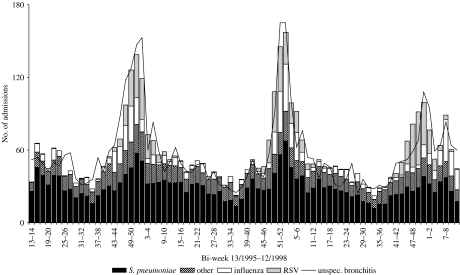
An adequate fit was also achieved for modelbronchitis and modelCOPD as indicated by an R2 of 0·80 and 0·78 respectively (Tables 4 and 5, and Figs 2 and 3). The final modelbronchitis contained the variables influenza virus, RSV, S. pneumoniae, and ‘other’, and for modelCOPD, whooping cough, H. influenzae, influenza virus, K. pneumoniae, RSV, S. pneumoniae, and streptococci other than S. pneumoniae remained in the final model. Table 5 shows the estimates of the proportion of unspecified bronchitis and unspecified COPD due to the specific respiratory pathogens.
Table 4.
Covariates in modelbronchitis
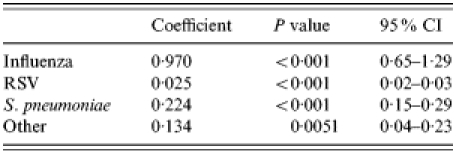
RSV, respiratory syncytial virus; CI, confidence interval.
Table 5.
Covariates in modelCOPD
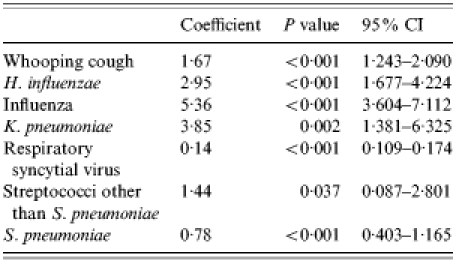
CI, confidence interval.
Fig. 2.
Comparison of observed bi-weekly number of unspecified bronchitis hospitalizations in the elderly (⩾65 years) with estimated number due to S. pneumoniae, influenza, respiratory syncytial virus (RSV), and other causes based on the final modelbronchitis shown in Table 4.
Fig. 3.
Comparison of observed bi-weekly number of unspecified COPD hospitalizations in the elderly (⩾65 years) with estimated number due to S. pneumoniae, H. influenzae, B. pertussis, influenza, other streptococci, respiratory syncytial virus (RSV), and K. pneumoniae based on the final modelCOPD shown in Table 5.
Table 3.
Estimates of the proportion of unspecified pneumonia, bronchitis and chronic pulmonary disease hospitalizations in the elderly (⩾65 years) due to specific respiratory pathogens

We excluded the intercept in all three models as the variable ‘other’ accounted for organisms not included as independent variables and for non-infectious causes. There was little change in the remaining estimated proportions and the model fit was almost the same.
Hospitalization rates
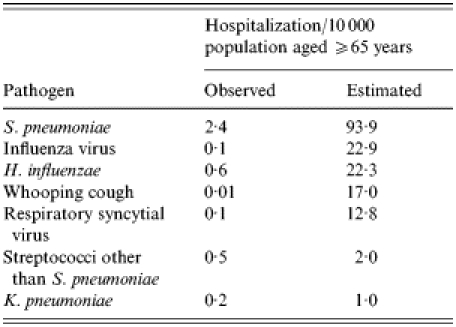
Applying the results of modelpneumonia, modelbronchitis and modelCOPD, the number of unspecified pneumonia, unspecified bronchitis and unspecified COPD hospitalizations in the elderly were calculated for each pathogen for the 3-year study period. Derived from these estimates the mean annual incidence of hospital admissions attributable to S. pneumoniae, influenza virus, H. influenzae, whooping cough, RSV, streptococci other than S. pneumoniae, and K. pneumoniae were calculated. Table 6 shows the observed and estimated LRI-associated hospitalization rates.
Table 6.
Comparison of observed and estimated lower respiratory-tract infection-associated hospitalization rates due to S. pneumoniae, influenza virus, H. influenzae, whooping cough, RSV, streptococci other than S. pneumoniae, and K. pneumoniae in the elderly (⩾65 years)
DISCUSSION
The method was applied successfully to generate robust models which indicate that the impact of the major respiratory pathogens S. pneumoniae, influenza virus, whooping cough, and RSV is far greater than is routinely recorded. In particular, estimates of the potential benefits of pneumococcal vaccination based upon reported numbers of admissions will be gross under-estimates.
These methods have the advantage over laboratory data of being potentially more sensitive. This is because diagnostic tests have limited sensitivity and clinicians do not always carry out appropriate investigations such as blood cultures or virology [13, 14]. Although apparently more sensitive, this analysis cannot replace laboratory-based surveillance which remains vitally important. First, availability of HES is not timely enough for surveillance, and the data are unwieldy to analyse, so could not be used to generate warning of epidemics of influenza or RSV, for example. Second, this method cannot distinguish subtypes of an organism such as the pneumococcal serotypes or different influenza strains if they display identical seasonality. Finally, a newly discovered pathogen which has identical seasonality to another known organism would not be detected by this method, and could only be identified through laboratory investigation [15].
The results indicate different predominance of different organisms in different clinical syndromes which mirrors clinical observation [16] and lends validity to the model. Haemophilus, Klebsiella, and non-pneumococcal streptococci contribute more greatly to COPD and bronchitis than pneumonia. These organisms are well recognized as causing exacerbations of chronic respiratory disease. Pertussis is increasingly recognized as causing atypical respiratory illness in adults, and this study indicates that it may have a significant role in causing hospitalization for pneumonia in elderly patients. In addition, different organisms have different levels of under-diagnosis in different syndromes as shown by the coefficients of the covariates in the models. Thus, the models indicate areas to target for improved methods of investigation and diagnostic sensitivity. Influenza seems to be responsible for around the same percentage of admissions for all three syndromes. Future analyses should be carried out to investigate the impact of recent changes in influenza vaccination policy.
The observed organism-specific rates of hospitalization show the pneumococcus as the most important cause of LRI overall followed by H. influenzae. The rates estimated from the modelling also place the pneumococcus at the top but move influenza from fifth to second in the ranking, and move pertussis up from bottom to fourth place. Furthermore, they stress the importance of RSV infection in the elderly, and the need for an effective RSV vaccine with hospitalization rates similar to those found recently by Falsey et al. [17]. These findings indicate the value of this approach which improves the estimates of burden and also changes the order of priorities for intervention.
For some pathogens, e.g. the pneumococcus, LRI is only one of the clinical syndromes caused. Others include septicaemia and meningitis. In addition milder community infections occur which do not result in hospital admission. The estimates of LRI hospitalizations, therefore, represent a part of the impact of these organisms on health.
Although our results give a good indication of the potential benefits of vaccination they do not indicate the best vaccination policy. Organisms such as influenza and pneumococcus have well-established interactions [18] and vaccination for one may have additional benefits in preventing a proportion of another infection which is not estimable from this analysis. In addition the impact of vaccination for a subgroup of types, as proposed for the pneumococcus, may have unpredictable results [19].
Mycoplasma did not appear in the final models against expectations. This may be explained by the fact that the 3 years of the study did not include an epidemic year and the seasonality of Mycoplasma in inter-epidemic years may not be strong enough to appear in the model separately from the background rate of LRI (‘other’).
This picture of LRI in the elderly shows that there is enormous potential to improve health using a few existing vaccines just for this particular clinical syndrome. The number of pathogens accounting for most LRI appears to be small, and for the majority of the organisms vaccines are already available (influenza, pneumococcus, pertussis) or under development (RSV). The next challenges are to define the most cost-effective design of new vaccination programmes, and decide how they could best be implemented.
ACKNOWLEDGEMENTS
The authors thank Dr Richard Pebody for critically reviewing this manuscript.
DECLARATION OF INTEREST
None.
REFERENCES
- 1.Falsey AR et al. Viral respiratory infections in institutionalized elderly: clinical and epidemiological findings. Journal of the American Geriatrics Society. 2001;40:115–119. doi: 10.1111/j.1532-5415.1992.tb01929.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Han LL, James PA, Anderson LJ. Respiratory syncytial virus pneumonia among the elderly: an assessment of disease burden. Journal of Infectious Diseases. 1999;179:25–30. doi: 10.1086/314567. [DOI] [PubMed] [Google Scholar]
- 3.Sorvillo FJ et al. An outbreak of respiratory syncytial virus pneumonia in a nursing home for the elderly. Journal of Infection. 1984;9:252–256. doi: 10.1016/s0163-4453(84)90530-9. [DOI] [PubMed] [Google Scholar]
- 4.Güris D et al. Changing epidemiology of pertussis in the United States: increasing reported incidence among adolescents and adults, 1990–1996. Clinical Infectious Diseases. 1999;28:1230–1237. doi: 10.1086/514776. [DOI] [PubMed] [Google Scholar]
- 5.Duthie EH, Abbasi AA. Laboratory testing: current recommendations for older adults. Geriatrics. 1991;46:41–45. , 49–50. [PubMed] [Google Scholar]
- 6.Dudas RA, Karron RA. Respiratory syncytial virus vaccines. Clinical Microbiology Reviews. 1998;11:430–439. doi: 10.1128/cmr.11.3.430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Department of Health http://www.dh.gov.uk/PolicyAndGuidance/HealthAndSocialCareTopics/Flu/FluGeneralInformation/fs/en. http://www.dh.gov.uk/PolicyAndGuidance/HealthAndSocialCareTopics/Flu/FluGeneralInformation/fs/en . Flu key documents ( ). Accessed 4 May 2005.
- 8.Johnson S et al. Development of a humanized monoclonal antibody (MEDI-493) with potent in vitro and in vivo activity against respiratory syncytial virus (RSV) Journal of Infectious Diseases. 1997;176:1215–1224. doi: 10.1086/514115. [DOI] [PubMed] [Google Scholar]
- 9.Marston BJ et al. Incidence of community-acquired pneumonia requiring hospitalization. Archives of Internal Medicine. 1997;157:1709–1718. [PubMed] [Google Scholar]
- 10.Crowcroft NS, Cutts F, Zambon MC. Respiratory syncytial virus: an underestimated cause of respiratory infection, with prospects for a vaccine. Communicable Diseases and Public Health. 1999;2:234–241. [PubMed] [Google Scholar]
- 11.Ryan MJ et al. Hospital admissions attributable to rotavirus infection in England and Wales. Journal of Infectious Diseases. 1996;174:S12–S18. doi: 10.1093/infdis/174.supplement_1.s12. (Suppl. 1): [DOI] [PubMed] [Google Scholar]
- 12.Müller-Pebody B et al. Contribution of RSV to bronchiolitis and pneumonia-associated hospitalisations in English children, April 1995–March 1998. Epidemiology and Infection. 2002;129:99–106. doi: 10.1017/s095026880200729x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Smith MD et al. Invasive pneumococcal infection in South and West England. Epidemiology and Infection. 1998;120:117–123. doi: 10.1017/s0950268897008522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fleming DM, Cross KW. Respiratory syncytial virus or influenza? Lancet. 1993;342:1507–1510. doi: 10.1016/s0140-6736(05)80082-0. [DOI] [PubMed] [Google Scholar]
- 15.Van den Hoogen BG et al. A newly discovered human pneumovirus isolated from young children with respiratory tract disease. Nature Medicine. 2001;7:719–724. doi: 10.1038/89098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lim WS et al. Study of community acquired pneumonia aetiology (SCAPA) in adults admitted to hospital: implications for management guidelines. Thorax. 2001;56:296–301. doi: 10.1136/thorax.56.4.296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Falsey AR et al. Respiratory syncytial virus infection in elderly and high-risk adults. New England Journal of Medicine. 2005;352:1749–1759. doi: 10.1056/NEJMoa043951. [DOI] [PubMed] [Google Scholar]
- 18.Butler JC, Schuchat A. Epidemiology of pneumococcal infections in the elderly. Drugs and Aging. 1999;15:11–19. doi: 10.2165/00002512-199915001-00002. (Suppl. 1): [DOI] [PubMed] [Google Scholar]
- 19.Gupta S, Ferguson N, Anderson R. Chaos, persistence, and evolution of strain structure in antigenically diverse infectious agents. Science. 1998;280:912–915. doi: 10.1126/science.280.5365.912. [DOI] [PubMed] [Google Scholar]