SUMMARY
We reviewed the epidemiological and microbiological characteristics of 89 reported outbreaks of waterborne infectious intestinal disease affecting 4321 people in England and Wales over the period 1992–2003. Public water supplies were implicated in 24 outbreaks (27%), private water supplies in 25 (28%), swimming pools in 35 (39%) and other sources in five outbreaks (6%). Cryptosporidium was implicated in 69% of outbreaks, Campylobacter sp. in 14%, Giardia in 2%, E. coli O157 in 3% and Astrovirus in 1%. From 2000, there was a consistent decline in the number of outbreaks of waterborne disease associated with public water supplies. The incidence rate of outbreaks in recipients of private water supplies may be as high as 35 times the rate in those receiving public water supplies (1830 vs. 53 per million population). Private water suppliers need to be aware of the importance of adequate treatment and the prevention of faecal contamination of storage water. Swimming-pool operators need to ensure chlorination and in particular adequate filtration measures are in place.
INTRODUCTION
Access to safe drinking water is a basic human right and an essential component of effective policy for health protection. Most health-related water-quality problems are the result of microbial contamination [1]. To ensure potable water, treatment is required and is a series of steps consisting of pre-treatment, mixing, coagulation, flocculation, settlement, filtration and disinfection [1].
Access to clean water is not just an issue for developing countries. Despite wealthy economies and access to proven drinking water-treatment technologies significant outbreaks of waterborne intestinal disease have occurred in North America and Western Europe over the last 10–15 years [2–7].
Outbreaks of waterborne infection in the United Kingdom have been previously reported [8–12]. Before 1980 typhoid, paratyphoid and dysentery were the main diseases associated with waterborne outbreaks but the improvements in water treatment, chlorination in particular, were highly effective in eliminating bacterial enteric pathogens [12]. Recognition and routine testing for Cryptosporidium and Campylobacter have only been undertaken in the last 30 years, and these organisms together with Giardia have now emerged as the enteric pathogens most frequently associated with waterborne outbreaks in England and Wales [13, 14].
In England and Wales most people (around 53 million or 99·5%) receive their domestic drinking water through public or mains water supplies provided by statutorily appointed water undertakers [15]. The UK Drinking Water Inspectorate regulates these companies. Private water supplies are those provided by someone other than statutorily appointed undertakers. Private water supplies are not regulated by the Drinking Water Inspectorate but are the responsibility of local authority environmental health departments, which must register the supplies, monitor them by chemical and microbiological analysis of water samples and approve them [16, 17].
The Communicable Disease Surveillance Centre (CDSC) and local health authorities in England and Wales have conducted structured surveillance of outbreaks of infectious intestinal disease (IID) since 1992 [18]. We reviewed the epidemiological and microbiological features of the subset of outbreaks of IID in which water was the reported vehicle of transmission in England and Wales in the period 1992–2003 utilizing the CDSC classification system for categorizing the strength of association with water [19].
METHODS
Surveillance
The CDSC, in collaboration with Consultants in Communicable Disease Control (CCDCs), introduced enhanced surveillance for outbreaks of IID in 1992. There was no statutory obligation to report but CCDCs, regional epidemiologists, environmental health officers, and clinical microbiologists were encouraged to telephone the CDSC Gastrointestinal Department with details, however preliminary, of any outbreaks under investigation. In addition outbreaks were detected through analysis of laboratory reporting databases, and national or localized increases in the reporting of particular pathogens were followed up.
Data collection and analysis
When a report was received from any source CDSC sent a questionnaire to the lead investigator. The questionnaire collected information on the total number of cases, number admitted to hospital, causative organism, specific water source, public/private water source, and evidence of failure of water quality by presence of indicator organisms. Data from returned questionnaires and follow-up reports were entered onto an MS Access database (Microsoft, Seattle, WA, USA). Important definitions used in the surveillance of IID are given in Table 1.
Table 1.
Definitions used in the surveillance of waterborne outbreaks

Information on waterborne outbreaks of IID was extracted from the CDSC MS Access database for analysis on 31 December 2003.
Cryptosporidium genotyping
Cryptosporidum can cause infectious diarrhoea in both livestock and humans. In England and Wales most isolates are characterized as genotype 1 which infects only humans and genotype 2 which infects both livestock and humans [20]. Cryptosporidium-positive faecal specimens from waterborne outbreaks, submitted to the Cryptosporidum Reference Unit, NPHS Microbiology Swansea were genotyped to identify species using PCR and RFLP analysis of a region of the Cryptosporidium oocyst wall protein gene. Data were available for the period 2000–2003 and cross-referenced with CDSC waterborne outbreak data.
Private water supplies
About 300 000 people live in households served by a private water supply [16]. There are about 50 000 registered private water supplies in England and Wales, of which 30 000 serve single dwellings [16]. Private water supplies are divided into Category One supplies where water is used for wholly domestic purposes and Category Two supplies for commercial food production and drinking water provision [21]. The total number of people exposed to a private water supply is likely to be higher than the domestic household figure as people from the wider population are unknowingly exposed particularly to private Category Two supplies in hospitals, hotels, restaurants, holiday homes and campsites [22].
Public water supplies
A public water supply is one provided by a water company appointed by the Director General of Water Services, for the purposes of drinking, washing, cooking or food production. There are 26 water companies in England and Wales with statutory responsibility for the provision of water to around 52 million people [23].
Incidence of waterborne outbreaks in public and private water supplies
In calculating the incidence rates of gastrointestinal disease from both public and private water supplies the numerator used was the total number of cases of gastroenteritis associated with each outbreak.
In calculating rates for outbreaks associated with public water supplies the denominator used was the mid-1997 census population for England and Wales, i.e. 51 412 600. This was the mid-point of the study period.
In calculating rates for outbreaks associated with private water supplies two calculations were done using different denominators to estimate the likely minimum and maximum incidence rate of waterborne outbreaks associated with private water supplies. To calculate minimum incidence rates the denominator used for recipients of public and private water supplies was the mid-1997 census population for England and Wales, i.e. 51 412 600. To calculate maximum incidence rates the denominator used for recipients of private water supplies was 300 000, and 51 112 600 (51 412 600−300 000) was used as a denominator estimate for recipients of public water supplies. Rates were expressed per million population over the 11-year period 1992–2003.
Strength of association
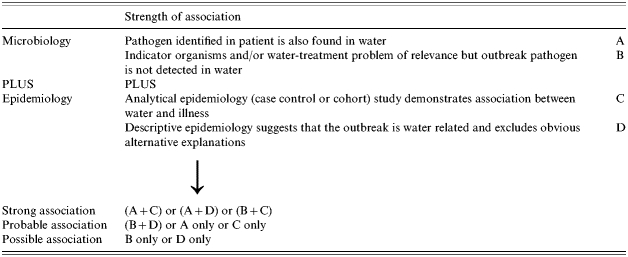
It can be difficult to prove beyond reasonable doubt that water is the associated cause of illness in an outbreak. CDSC developed a method of categorizing the degree of evidence used to implicate a water source. These categories take into account the epidemiology, microbiology and water-quality information available from the outbreak [19]. The scoring system is shown in Table 2.
Table 2.
Communicable Disease Surveillance Centre (CDSC) classification system for categorizing strength of association
RESULTS
Outbreaks
Between 1 January 1992 and 31 December 2003, CDSC received 89 waterborne IID outbreak reports affecting 4321 people.
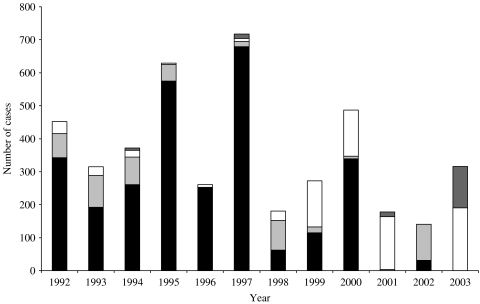
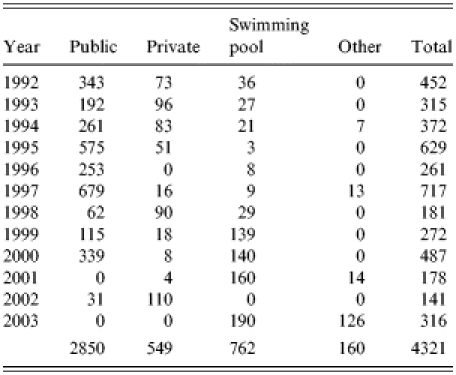
Public water supplies were implicated in 24 outbreaks (27%), private water supplies in 25 (28%), swimming pools in 35 (39%), and other sources in five outbreaks (6%), three involving recreational river use and two involving fountains. There was an average of 119 case patients per public water outbreak and 22 cases per private water outbreak (Fig. 1).
Fig. 1.
Total number of case patients associated with waterborne outbreaks of infectious intestinal disease.
 , Other water supplies; □, swimming pools;
, Other water supplies; □, swimming pools;
 , private water supplies; ■, public water supplies.
, private water supplies; ■, public water supplies.
The number of case patients associated with waterborne outbreaks involving public water supplies fell consistently, with a particularly dramatic decline since 2000. Outbreaks associated with private water supplies, on the other hand, increased in number (Fig. 1, Table 3).
Table 3.
Total number of case patients associated with waterborne outbreaks of infectious intestinal disease
Public vs. private water supplies
Annual incidence rates of gastroenteritis associated with private water supplies were considerably higher than those involving public water supplies: the overall rate for municipal public water supplies was 53 per million population for the period 1992–2003 compared with 1830 per million for private supplies during 1992–2003. There were no deaths recorded during this period.
Seasonality
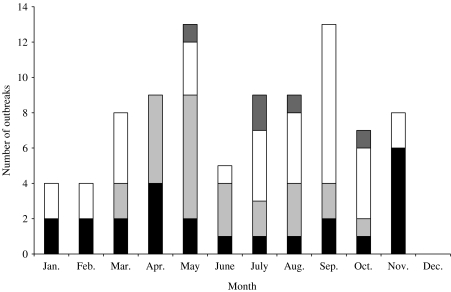
Outbreaks of waterborne IID involving public water supplies showed a bimodal distribution peaking in the spring (March–April) and late autumn (October–November). Those involving private water supplies showed a spring peak (April–May) with a gradual decline as the summer progressed. Outbreaks of waterborne IID involving swimming pools showed a late summer and autumn peak (July–October) (Fig. 2).
Fig. 2.
Seasonal pattern of waterborne outbreaks England and Wales 1992–2003.
 , Other; □, swimming pools;
, Other; □, swimming pools;
 , private; ■, public.
, private; ■, public.
Implicated organism
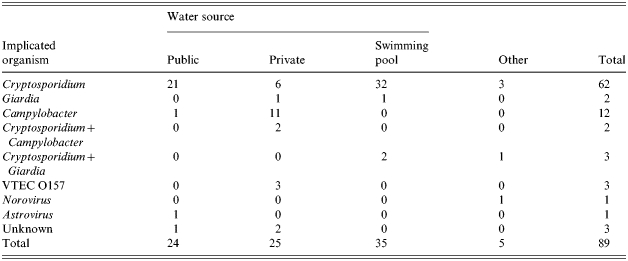
Cryptosporidium alone was implicated in 62 outbreaks (70%), Campylobacter spp. in 12 (14%), Giardia in two (2%), E. coli O157 (phage types 2, 4, 21/28) in three (3%), Astrovirus in one outbreak (1%), and Norovirus in one (1%). Cryptosporidium combined with Campylobacter spp. were implicated in two outbreaks (2%). Cryptosporidium combined with Giardia were implicated in three outbreaks (3%). There were three outbreaks of unknown cause. Thus, of the 86 outbreaks of known aetiology, Cryptosporidium was present in 67 (78%). The distribution by water source is shown in Table 4.
Table 4.
Implicated organism and water source in waterborne outbreaks 1992–2002
Cryptosporidium genotyping
The Cryptosporidium Reference Unit provided genotyping data on 12 of these outbreaks for the period 2000–2003. Cryptosporidium hominis (which is largely restricted to humans) was implicated in six outbreaks: two public supplies, one private supply and three swimming pools. Cryptosporidium parvum (which has a wide host range including livestock and humans) was implicated in four outbreaks: one public supply, one private supply and two swimming pools. One outbreak involved a combination of C. hominis and C. parvum (HPA, personal communication, 2004).
Strength of association
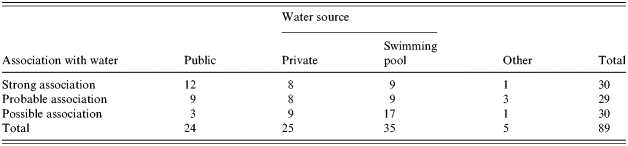
Unequivocal supportive microbiological evidence (Category A in Table 2) was obtained from implicated water samples in 27 (30·3%) outbreaks.
When microbiological and epidemiological evidence was combined 59 (66%) waterborne outbreaks were categorized as either having a strong or probable association with water (Table 5).
Table 5.
Strength of association of outbreaks with water and water sources 1992–2003
DISCUSSION
Private water supplies were implicated in 25 (28%) outbreaks in the period 1992–2003 in England and Wales. Campylobacter was implicated in 52% of these, which is in marked contrast with public water supplies, where a bacterial pathogen was present in only one outbreak. It is difficult to estimate the total number of people who are exposed to private water supplies. Most supplies usually serve small populations such as farms, institutions, and rural communities totalling about 300 000 people. However, it is likely that a much larger transient population are exposed to these supplies through hospitals, hotels, holiday homes and campsites. Since 2001 incidence rates and the number of case patients associated with waterborne outbreaks involving private water supplies has overtaken those associated with public water supplies. The overall incidence rate of waterborne outbreaks in recipients of private water supplies may be as high as 35 times the rate in those receiving public water supplies (1830 vs. 53 per million population).
Public water supplies were implicated in 24 (27%) outbreaks in the period 1992–2003 in England and Wales. Cryptosporidium was present in 21 (88%) of these. Cryptosporidium oocysts are resistant to chlorine at the levels used in water treatment and filtration remains the most effective method for their removal. The threat posed by Cryptosporidium has been the subject of three reports of an Expert Group on Cryptosporidium in Water Supplies [24–26]. Furthermore water companies in England and Wales are required to conduct risk assessments of their water sources for Cryptosporidium and since 1 April 2000 to undertake continual monitoring of treated water for oocysts at treatment plants considered to be at high risk from Cryptosporidium in the treated water [27]. This review has demonstrated that since the mid-1990s incidence rates and the number of case patients associated with waterborne outbreaks involving public water supplies have fallen consistently with a particularly dramatic decline since 2000.
Campylobacter has been the pathogen most frequently associated with private water supply outbreaks for over 30 years [28, 29]. Investigating Campylobacter outbreaks can prove difficult as more than one strain of Campylobacter and more than one pathogen may be isolated from a suspect source [15, 29]. This proved to be the case in two of the outbreaks (Table 1) when Cryptosporidium was also isolated. E. coli O157 was the implicated organism in three private water supply outbreaks. The most serious waterborne outbreak in recent times, the Walkerton outbreak in Canada in 2002, was caused by a combination of pathogens, E. coli O157 and Campylobacter [2].
A number of common themes emerged as possible contributory factors to the outbreaks reported. These included an inadequate or a transient failure of water treatment measures, overloading of the treatment process through gross contamination of the water source, contamination of water source with animal or human faeces, use of recreational pools by individuals with gastrointestinal symptoms.
Swimming pools were implicated in 35 outbreaks (39%) with Cryptosporidium implicated in 33 and Giardia in two. Guidance on management and maintenance is available for pool operators from the Pool Water Treatment Advisory Group [30]. While proper management and maintenance procedures should reduce the risk of prolonged contamination of swimming pools by Cryptosporidium oocysts it is difficult, if not impossible, to prevent point source outbreaks due to faecal contamination in the form of accidental faecal release especially from small children. Individuals and parents of young children should be reminded that the use of recreational water facilities by those with gastrointestinal symptoms should be avoided, as it is a potential threat to public health. Analytical studies are especially important in strengthening the evidence for an association with a swimming pool. Point source contamination of swimming pools is unlikely to persist long enough to be detectable by the time the outbreak is recognized and sampling of water arranged [14]. Normal pool water samples do not in themselves provide evidence to exclude pool water as a source of the outbreak, but if abnormal, provide strong evidence that it is. There is also the added difficulty of interpreting the significance of the presence of low numbers of Cryptospordium oocysts in filter backwash.
As Cryptosporidium is by far the most commonly implicated organism in waterborne outbreaks described here it is unsurprising that the seasonal distribution is consistent with that of human cryptosporidiosis. Spring peaks in reports of human cryptosporidiosis have been attributed to lambing, calving, the application of slurry combined with high rainfall leading to run-off from agricultural land into surface water and drinking water catchments [13, 31]. A late summer and early autumn peak of cryptosporidiosis is frequently observed in annual reports that may well be attributed to a summer travel phenomenon to countries with higher incidence or involving greater environmental exposure to the parasite and their subsequent importation and diagnosis on return to the United Kingdom. For example between January 2000 and December 2002 over 200 laboratory-confirmed cases of cryptosporidiosis were confirmed in English and Welsh holidaymakers returning from Spain largely clustered during the late summer of 2000 [32]. A similar large summer and autumn peak of cryptosporidiosis has also been reported in England and Wales in 2003 [33]. Swimming pool-associated outbreaks in England and Wales showed a different seasonal pattern from drinking waterborne outbreaks with the highest incidence in late summer and early autumn.
Genotyping of Cryptosporidium has provided much needed insight into the genus only some of which are infectious to humans. These methods can assist in determining the public health significance of oocysts present in water by identifying those species and genotypes that are human-infective and also contribute to the linking of cases in an outbreak to a common source.
Direct microbiological evidence of contamination of water supplies was only present in just over a third of reported outbreaks. This is not surprising as there are considerable difficulties associated with the microbiological examination of water in the context of outbreaks. By the time an outbreak is detected the relevant body of water may be long gone. For a pathogen to be detected it usually requires either very heavy contamination associated with a catastrophic breakdown of treatment measures or contamination that continues over a long period of time. And even when there is microbiological evidence of contamination it can be difficult to relate its presence to the presumed time of exposure of cases. The relative persistence of Cryptosporidium compared with other organisms does make it more likely to be implicated in outbreaks. However, the presence of Cryptosporidium oocysts for example only proves that they were able to penetrate the water treatment system. The absence of oocysts or any other pathogen only proves that they were absent in the sample taken, not that they were absent in the relevant water supply. The absence of microbiological evidence in the majority of waterborne outbreaks emphasizes the critical importance of well-conducted analytical and/or descriptive epidemiological studies to assess and characterize the risk associated with waterborne outbreaks. This also impacts on the strength of association of an outbreak with the suspected source. It is interesting that while 50% of outbreaks were strongly associated with municipal supplies, few swimming pool-associated outbreaks fell into this category with nearly half having a ‘possible’ association.
There was a consistent decline in the number of outbreaks of waterborne disease associated with public water supplies, particularly noticeable since 2000. Private water supplies, on the other hand, are an ongoing concern. The microbiological quality of many private water supplies is poor [34–36]. Outbreaks of waterborne disease associated with private water supplies increased in number during the period of the study. If a large private water supply becomes contaminated it can pose a substantial risk to public health [35]. We would suggest that the regulatory framework for private water supplies needs to be strengthened and that this should include an obligation on suppliers to inform recipients that they are consuming water from a private supply.
As no deaths were recorded between 1992 and 2003 and only 4321 individuals were reported to have symptoms, is the surveillance of waterborne outbreaks of IID necessary? This is a dangerous conclusion to make on the basis of numbers alone. People with gastrointestinal illness often do not seek medical attention and the safety of water supplies can fail unexpectedly with disastrous consequences [36]. In the spring of 1993 over 400 000 people in Milwaukee became ill, and over 4000 were hospitalized, because of municipal water contaminated with Cryptosporidium [5]. More recently an estimated 2300 people became seriously ill and seven died from exposure to drinking water contaminated with E. coli O157:H7 and Campylobacter fetus subsp. jejuni in the town of Walkerton, Ontario, Canada in 2000 [2].
Although there are undoubtedly circumstances specific to individual outbreaks there are some common themes running through waterborne outbreaks both in the United Kingdom and elsewhere. An inadequacy was often identified in the treatment provided, or in the operation of the treatment process, or where there was overloading of the treatment process [37]. The Commissioner of the Walkerton Inquiry accepted the expert evidence that a multiple barrier approach was necessary for providing safe drinking water taking account of the following main elements: the water source, its treatment and distribution, monitoring and responding appropriately to breaches in quality. Critical for this approach to work is that all these elements must be maintained effectively by requiring that the individual performances of statutorily appointed water companies, local authorities, primary care trusts, local and national health protection teams meet best practice criteria. The biggest barrier to achieving this is complacency.
The surveillance of waterborne IID is not without its difficulties. For example outbreaks at events where there is a well-defined cohort of people are more likely to be identified and investigated than those in which cases are widely dispersed. All of this contributes to difficulty in detecting an actual rise in cases against a background of ongoing gastrointestinal disease. Underreporting of cases and outbreaks are somewhat inevitable biases as detection is dependent on many factors including severity of symptoms, GP attendance, clinical sampling, laboratory confirmation, geographical spread and probably water source. Undoubtedly there are outbreaks which go undetected but the actual number of outbreaks which go undetected is difficult to quantify. Nevertheless it seems clear from the surveillance described in this and other papers that waterborne outbreaks of IID are still a problem in countries with highly developed sanitation systems, and that private water supplies are a particular hazard.
DECLARATION OF INTEREST
None.
REFERENCES
- 1.Binnie C Basic Water Treatment. 3rd edn. London: IWA Publishing Co.; 2002. [Google Scholar]
- 2.Hrudey SE et al. A fatal waterborne disease epidemic in Walkerton Ontario: comparison with other waterborne outbreaks in the developed world. Water Science and Technology. 2003;47:7–14. [PubMed] [Google Scholar]
- 3.Laursen EO et al. Gastroenteritis: a waterborne outbreak affecting 1,600 people in a small Danish town. Journal of Epidemiology and Community Health. 1994;48:453–458. doi: 10.1136/jech.48.5.453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bridgman SA et al. Outbreak of cryptosporidiosis associated with a disinfected groundwater supply. Epidemiology and Infection. 1995;115:555–566. doi: 10.1017/s0950268800058726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mackenzie WR et al. Massive outbreak of waterborne cryptosporidium infection in Milwaukee, Wisconsin: recurrence of illness and risk of secondary transmission. Clinical Infectious Diseases. 1995;21:57–62. doi: 10.1093/clinids/21.1.57. [DOI] [PubMed] [Google Scholar]
- 6.Angulo FJ et al. A community waterborne outbreak of salmonellosis and the effectiveness of a boil water order. American Journal of Public Health. 1997;87:580–584. doi: 10.2105/ajph.87.4.580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Stirling R et al. Waterborne cryptosporidiosis outbreak, North Battleford, Saskatchewan, Spring 2001. Canada Communicable Disease Report. 2001;27:185–192. [PubMed] [Google Scholar]
- 8.Stanwell-Smith R, Golding AMB, Noah N, Stanwell-Smith R. Water and Public Health. London: Smith-Gordon Nishimura; 1994. Water and public health in United Kingdom. Recent trends in the epidemiology of waterborne disease; pp. 39–54. , pp. [Google Scholar]
- 9.Meinhardt PL, Casemore DP, Miller KB. Epidemiological aspects of human cryptosporidiosis and the role of waterborne transmission. Epidemiologic Reviews. 1997;18:118–136. doi: 10.1093/oxfordjournals.epirev.a017920. [DOI] [PubMed] [Google Scholar]
- 10.Furtado C et al. Outbreaks of waterborne infectious intestinal disease in England and Wales, 1992–1995. Epidemiology and Infection. 1998;121:109–119. doi: 10.1017/s0950268898001083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Galbraith NS, Barrett NJ, Stanwell-Smith R. Water and disease after Croydon: a review of water associated disease in the UK 1937–86. Journal of the Institution of Water & Environmental Management. 1987;1:7–21. [Google Scholar]
- 12.Galbraith NS, Golding AMB, Noah N, Stanwell-Smith R. Water and Public Health. Great Britain; 1994. Historical review of microbial disease spread by water in England and Wales; pp. 15–37. , pp. [Google Scholar]
- 13.Stanwell-Smith R, Golding AMB, Noah N, Stanwell-Smith R. Water and Public Health. Great Britain; 1994. Water and public health in United Kingdom. Recent trends in the epidemiology of water borne disease; pp. 39–54. , pp. [Google Scholar]
- 14.Meinhardt PL, Casemore DP, Miller KB. Epidemiological aspects of human cryptosporidiosis and the role of waterborne transmission. Epidemiologic Reviews. 1997;18:118–136. doi: 10.1093/oxfordjournals.epirev.a017920. [DOI] [PubMed] [Google Scholar]
- 15.Said B et al. Outbreaks of infectious disease associated with private drinking water supplies in England and Wales 1970–2000. Epidemiology and Infection. 2003;130:469–479. [PMC free article] [PubMed] [Google Scholar]
- 16.Drinking Water Inspectorate. Drinking Water 1998. London: Her Majesty's Stationery Office; 1998. Private water supplies, chapter 4. [Google Scholar]
- 17.Drury D. Private water supplies: classification and monitoring. Communicable Disease Report 1995;5:R98–R99. . CDR Review . [PubMed] [Google Scholar]
- 18.Wall PG et al. Food poisoning: notifications, laboratory reports, and outbreaks-where do the statistics come from and what do they mean? Communicable Disease Report. CDR Weekly. 1996;6:R93–R100. [PubMed] [Google Scholar]
- 19.Tillett HE, de Louvois J, Wall PG. Surveillance of outbreaks of waterborne infectious disease: categorising levels of evidence. Epidemiology and Infection. 1998;120:37–42. doi: 10.1017/s0950268897008431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McLaughlin J et al. Molecular epidemiological analysis of Cryptosporidium spp. in the United Kingdom; results of genotyping in 1,705 fecal samples from humans and 106 fecal samples from livestock anaimals. Journal of Clinical Microbiology. 2000;38:3984–3990. doi: 10.1128/jcm.38.11.3984-3990.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.The Private Water Supplies Regulation 1991. Statutory Instrument No. 2790. London: Her Majesty's Stationery Office; [Google Scholar]
- 22.Shepherd KM, Wyn-Jones AP. Private water supplies and the local authority role; results of a UK national survey. Water Science and Technology. 1997;35:41–45. [Google Scholar]
- 23.Drinking Water Inspectorate. Drinking Water 2003. Annual Report for England and Wales. www.dwi.gov.uk/reports.shtm www.dwi.gov.uk/reports.shtm
- 24.Expert Group on Cryptosporidium in Water Supplies. Cryptosporidium in water supplies: report of the group of experts: chairman Sir John Badenoch. London: Her Majesty's Stationery Office; 1990. [Google Scholar]
- 25.Expert Group on Cryptosporidium in Water Supplies. Cryptosporidium in water supplies: report of the group of experts: chairman Sir John Badenoch. London: Her Majesty's Stationery Office; 1995. [Google Scholar]
- 26.Expert Group on Cryptosporidium in Water Supplies. Cryptosporidium in water supplies: report of the group of experts: chairman Professor Ian Bouchier. London: Her Majesty's Stationery Office; 1998. [Google Scholar]
- 27.The Water Supply (Water Quality) (Amendment) Regulations 1999 1999. . Statutory Instrument . , No. 1524.
- 28.Frost JA, Gillespie IA, O'Brien SJ. Public health implications of campylobacter outbreaks in England and Wales, 1995–9: epidemiological and microbiological investigations. Epidemiology and Infection. 2002;128:111–118. doi: 10.1017/s0950268802006799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Duke LA et al. A mixed outbreak of cryptosporidium and campylobacter infection associated with a private water supply. Epidemiology and Infection. 1996;116:303–308. doi: 10.1017/s0950268800052614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pool Water Treatment Advisory Group. Swimming Pool Water: Treatment and quality standards. Greenhouse Publishing; 1999. [Google Scholar]
- 31.Smerdon WJ et al. Foot and Mouth disease in livestock and reduced cryptosporidiosis in humans, England and Wales. Emerging Infectious Diseases. 2003;9:22–28. doi: 10.3201/eid0901.020512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Anon. British tourists return from Majorca with cryptosporidiosis. Communicable Disease Report. CDR Weekly. 2000;10:285. [Google Scholar]
- 33.Anon . Large summer and autumn peak of cryptosporidiosis in England and Wales 2003. Communicable Disease Report CDR Weekly200313: news. (http://www.hpa.org.uk/cdr/PDFfiles/2003/cdr4103.pdf
- 34.Rutter M et al. A survey of the microbiological quality of private water supplies in England. Epidemiology and Infection. 2000;124:417–425. doi: 10.1017/s0950268899003970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Drinking Water 2002 London: Her Majesty's Stationery Office; 2003. . A report by the Chief Inspector Drinking Water Inspectorate. [Google Scholar]
- 36.Reacher M et al. Outbreak of gastroenteritis associated with contamination of a private borehole water supply. Communicable Disease and Public Health. 1999;2:27–31. [PubMed] [Google Scholar]
- 37.Infectious Intestinal Disease Study Teams Food Standards Agency; London: HMSO; 2000. . A report of the study of infectious intestinal disease in England. [Google Scholar]